��Ŀ����
ij��Ԫ�ᣨH2A������ʽ�������룺H2A��H++HA-��HA- H++A2-����������������Һ��0��0lmol/L��H2A��Һ��0��0lmol/L��NaHA��Һ
��0��02mol/L��HC1��Һ��0��04mol/L��NaHA��Һ�������Ϣ�0��02mol/L��NaOH��Һ��0��02mol/L��NaHA��Һ��������
H++A2-����������������Һ��0��0lmol/L��H2A��Һ��0��0lmol/L��NaHA��Һ
��0��02mol/L��HC1��Һ��0��04mol/L��NaHA��Һ�������Ϣ�0��02mol/L��NaOH��Һ��0��02mol/L��NaHA��Һ��������
���й�������������Һ��˵����ȷ���� �� ��
A����Һ���д���ˮ��ƽ�⣺HA-+H2O H2A+OH-
H2A+OH-
B����Һ�����У�c��HA-��+c��A2һ��=c��Na+��
C����Һ�����У�c��OHһ��=c��H+�� +c��HA-�� +2c��H2A��
D��������Һ��c��HA-��Ũ�ȴ�С����>��>��>��
���𰸡�
B
����������

��ϰ��ϵ�д�
�����Ŀ
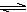 H++A2������������������Һ��
H++A2������������������Һ��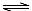 H2A+OH�D
H2A+OH�D H++A2������������������Һ��˵����ȷ����
H++A2������������������Һ��˵����ȷ����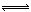 H++A2������������������Һ��˵����ȷ����
( )
H++A2������������������Һ��˵����ȷ����
( )