��Ŀ����
ij��Ԫ�ᣨH2A������ʽ�������룺H2A��H++HA����HA��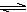 H++A2������������������Һ��
H++A2������������������Һ��
�� 0.01mol?L1��H2A��Һ
�� 0.01mol?L1��NaHA��Һ
�� 0.02mol?L1��HCl��Һ��0.04mol?L1��NaHA��Һ��������
�� 0.02mol?L1��NaOH��Һ��0.02mol?L1��NaHA��Һ��������
���й�������������Һ��˵����ȷ���ǣ� ��
A����Һ���д���ˮ��ƽ�⣺HA�D+H2O 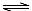 H2A+OH�D
H2A+OH�D
B����Һ�����У�c(HA��)+2c(A2��)=c(Na+)
C����Һ�����У�c(OH��)=c(H+)+c(HA��)
D��������Һ��c (HA��)Ũ�ȴ�С���ۣ��٣��ڣ���
CD
��������
�����������Ԫ��H2A��ˮ��Һ�з������룺H2A��H++HA-��HA- H++A2-����˵���Ƕ�Ԫ��ĵ�һ����������ȫ�ģ����ڶ���������ڵ���ƽ�⡣���HA-����ֻ���ڵ���ƽ�⣬������ˮ��ƽ�⣬��Һ�����ԣ�ѡ��A����ȷ��B����ȷ�������������غ㣬Ӧ����c(HA��)+c(A2��)=c(Na+)������ǡ�÷�Ӧ����Na2A��ˮ����Һ�Լ��ԣ���ȷ�����������غ㣻��������Ĵ�������HA���ĵ��룬c (HA��)Ũ�����������С���ٵ�һ������������������Ƶڶ����ĵ��룬����c (HA��)Ũ�ȴ��ڢ��У���ѡ��D��ȷ����ѡCD��
H++A2-����˵���Ƕ�Ԫ��ĵ�һ����������ȫ�ģ����ڶ���������ڵ���ƽ�⡣���HA-����ֻ���ڵ���ƽ�⣬������ˮ��ƽ�⣬��Һ�����ԣ�ѡ��A����ȷ��B����ȷ�������������غ㣬Ӧ����c(HA��)+c(A2��)=c(Na+)������ǡ�÷�Ӧ����Na2A��ˮ����Һ�Լ��ԣ���ȷ�����������غ㣻��������Ĵ�������HA���ĵ��룬c (HA��)Ũ�����������С���ٵ�һ������������������Ƶڶ����ĵ��룬����c (HA��)Ũ�ȴ��ڢ��У���ѡ��D��ȷ����ѡCD��
���㣺����������ˮ���Ӧ�ã�����Ũ�ȴ�С�ıȽ�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������߿����ۺ���ǿ����ע�ض�ѧ������֪ʶ������ѵ����ͬʱ�����ض�ѧ����������������ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������ͷ�ɢ˼ά����������Ĺؼ������úü����غ��ϵ��������غ㡢�����غ��Լ������غ㣬��λ���Ҫע����ʽ�εĵ���̶Ⱥ�ˮ��̶ȵ���Դ�С��

 H++A2-����������������Һ��0��0lmol/L��H2A��Һ��0��0lmol/L��NaHA��Һ
��0��02mol/L��HC1��Һ��0��04mol/L��NaHA��Һ�������Ϣ�0��02mol/L��NaOH��Һ��0��02mol/L��NaHA��Һ��������
H++A2-����������������Һ��0��0lmol/L��H2A��Һ��0��0lmol/L��NaHA��Һ
��0��02mol/L��HC1��Һ��0��04mol/L��NaHA��Һ�������Ϣ�0��02mol/L��NaOH��Һ��0��02mol/L��NaHA��Һ�������� H++A2������������������Һ��˵����ȷ����
H++A2������������������Һ��˵����ȷ����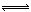 H++A2������������������Һ��˵����ȷ����
( )
H++A2������������������Һ��˵����ȷ����
( )