��Ŀ����
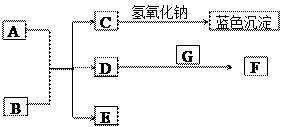
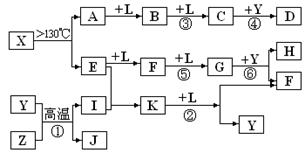
��ͼ��A~G��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ�������£�AΪ�Ϻ�ɫ��������,BΪ������ǿ�ᣬGΪ�ؿ��к������Ԫ����ɵ���̬���ʣ�D��F���Ǵ�����Ⱦ���Ҫ��Դ������β����DΪ��ɫ���壬FΪ����ɫ���塣
��ش��������⣺
��D�Ļ�ѧʽ�� ��C+NaOH���ɵ���ɫ������ ���ѧʽ����
�ڹ���G���ʵ�Ԫ����Ԫ�����ڱ��е�λ��Ϊ ��
��д��B��ϡ��Һ��A��Ӧ�Ļ�ѧ����ʽ�� ��

��ش��������⣺
��D�Ļ�ѧʽ�� ��C+NaOH���ɵ���ɫ������ ���ѧʽ����
�ڹ���G���ʵ�Ԫ����Ԫ�����ڱ��е�λ��Ϊ ��
��д��B��ϡ��Һ��A��Ӧ�Ļ�ѧ����ʽ�� ��
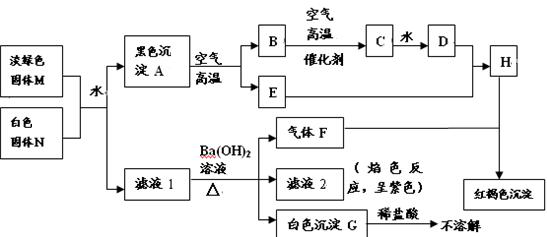
��NO�� Cu(OH)2 �ڵڶ����ڢ�A���3Cu+8HNO3(ϡ)=3Cu(NO3)2+2NO��+4H2O
��

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ

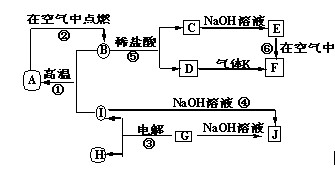
 ��
��