��Ŀ����
������Ԫ��X��Y��Z��W�����ڱ��е�λ�ù�ϵ����ͼ��ʾ����֪��ͬ����Ԫ�صij����������У�W�ļ����Ӱ뾶��С��X��Y��Z��W�ĵ��ʼ��仯�������ճ���������;����㷺��
| | X | Y | Z |
| W | | | |
��1��XԪ����Ԫ�����ڱ��е�λ��___ __________��
��2��X��Y��ZԪ�ص��⻯��������ֻ��������ϣ�����һ�������£�Һ̬YH3��Һ̬H2Z���Է������Ʒ�ʽ���룬��Һ̬YH3�������ӵĵ���ʽΪ_______________��
��3����ϸWY��ĩ��Ӧ���ڴ��ģ���ɵ�·����������ԭ��ΪW2Z3��Y2��X�ڸ����·�Ӧ�������ֻ���������ֻ������������Ԫ����ɣ���ԭ�Ӹ����Ⱦ�Ϊ1��1���䷴Ӧ�Ļ�ѧ����ʽΪ _��
��4����WΪ�����Ƴɵ������ڿ����о������ұ������ã���������____����ܡ����ܡ������������̲ˣ�ԭ����_________________________________________��
��5��ij����β����������ȼ�ϵ��Ϊ����ԭ���ⶨXZ��Ũ�ȣ���װ����ͼ��ʾ���õ���е����Ϊ�����ƣ������ƣ�����Z2-�����ڹ������NASICON�������ƶ������ķ�Ӧʽ___________��

���ڸõ�ص�����˵������ȷ����______
A������ʱ�缫b��������Z2- ͨ���������NASICON�ɵ缫b����缫a
B������ʱ�����ɵ缫aͨ������������缫b
C����������ͨ���ĵ���Խ��β����XZ�ĺ���Խ��
��6����Ԫ��X��Ԫ��Z��ɵ�ij�������Ӿ��л�ԭ�ԣ��ܱ�����KMnO4����������д��Ӧ�����ӣ���������ƽ��
 ��
��
��1���ڶ�����2�����ڢ�A �壨2�֣� ��2��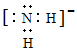 ��2�֣�
��2�֣�
��3��Al2O3 + N2 + 3C  2AlN + 3CO��2�֣�
2AlN + 3CO��2�֣�
��4�����ܣ�1 �֣��������ӻ��ƻ�����������Ĥ��2�֣�
��5��CO + O2-�D2e-��CO2��2�֣���AC��2�֣�
��6��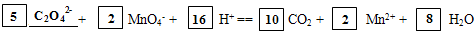 ��2�֣�
��2�֣�
�������������������Ԫ��X��Y��Z��W�����ڱ��е�λ�ù�ϵ��ͼ��ʾ����֪��ͬ����Ԫ�صij����������У�W�ļ����Ӱ뾶��С������Ԫ�ص����λ�ÿ�֪��WӦ���ǵ�������Ԫ�أ�����Wһ������Ԫ�أ���X��Y��Z�ֱ���C��N��O��
��1��̼Ԫ����Ԫ�����ڱ��е�λ���ǵڶ����ڵڢ�A �塣
��2��һ�������£�Һ̬YH3��Һ̬H2Z���Է������Ʒ�ʽ���룬�����ˮ�ĵ��뷽��ʽH2O��H2O H3O����OH����֪��Һ̬YH3�ĵ��뷽��ʽΪNH3��NH3
H3O����OH����֪��Һ̬YH3�ĵ��뷽��ʽΪNH3��NH3 NH4����NH2������������ӵĵ���ʽΪ
NH4����NH2������������ӵĵ���ʽΪ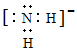 ��
��
��3������ԭ��ΪW2Z3��Y2��X�ڸ����·�Ӧ�������ֻ���������ֻ������������Ԫ����ɣ���ԭ�Ӹ����Ⱦ�Ϊ1��1��������ԭ���غ��֪�����ֻ�����Ӧ����AlN��CO�������䷴Ӧ�Ļ�ѧ����ʽΪAl2O3 + N2 + 3C  2AlN + 3CO��
2AlN + 3CO��
��4�������̲��к��д����������ӣ��������ӻ��ƻ�����������Ĥ�����Ը������������������̲ˡ�
��5��ԭ����нϻ��õĽ����Ǹ�����ʧȥ���ӣ�����������Ӧ�����Ӿ����ߴ��ݵ�������������Һ�е��������������ƶ��������õ����ӣ�������ԭ��Ӧ�������ڸ�ԭ�����CO�ڸ���ͨ�뷢��������Ӧ����CO2������������ͨ�������õ���������O2-��O2-���������������Ը����缫��ӦʽΪCO + O2-�D2e-��CO2��A������ʱ�缫b��������Z2- ͨ���������NASICON�ɵ缫b����缫a��A��ȷ��B������ʱ�����ɵ缫bͨ������������缫a��B����ȷ��C����������ͨ���ĵ���Խ�����ĵ�COԽ�࣬��˵��β����XZ�ĺ���Խ�ߣ�C��ȷ����ѡAC��
��6��Ԫ��X��Ԫ��Z��ɵ�ij�������Ӿ��л�ԭ�ԣ��ܱ�����KMnO4����������������C2O42������Ӧ��̼Ԫ�صĻ��ϼ۴�+3�����ߵ�+4��ʧȥ1�����ӣ���MnԪ�صĻ��ϼ۴�+7�۽��͵�+2�۵õ�5�����ӣ���˸��ݵ��ӵ�ʧ�غ��֪�������뻹ԭ�������ʵ���֮����2:5�������ԭ���غ��֪����ƽ��ķ���ʽΪ5C2O42����2MnO4����16H��=10CO2��2Mn2����8H2O��
���㣺����Ԫ���ƶϡ�����ʽ��������ԭ��Ӧ��ƽ�������ĸ�ʴ�Լ��绯ѧԭ�����й��ж�

 С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д� ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
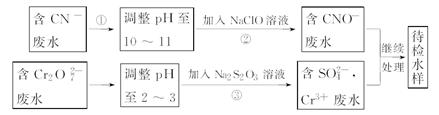
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�ij�������Ƹ﹤ҵ������CrԪ�صĻ����������ù������£������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe2+��Fe3+��Al3+��Cu2+��Mg2+����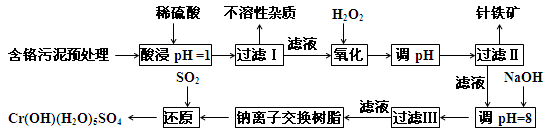
�����²������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe3+ | Fe2+ | Mg2+ | Al3+ | Cu2+ | Cr3+ |
| ��ʼ����ʱ��pH | 1.9 | 7.0 | 9.3 | 3.7 | 4.7 | --- |
| ������ȫʱ��pH | 3.2 | 9.0 | 11.1 | 8.0 | 6.7 | 9(>9 �ܽ�) |
��1�����ʱ��Ϊ����߽�ȡЧ�ʿɲ�ȡ�Ĵ�ʩ��____________________������дһ������
��2������H2O2 Ŀ��������_______���ӣ����йص����ӷ���ʽ______________________��������Coethite�����Ե¹�ʫ�˸�£�Coethe�����������ģ����Ԫ����Fe��H��O����ѧʽ��Ϊ89���仯ѧʽ��______________��
��3����pH=8��Ϊ�˽�_____________���ӣ���Fe3+��Al3+��Cu2+��Mg2+��ѡ�������������������ʽ��ȥ���˳��ij����в��ֳ������ܽ�������������������Һ�У����йص����ӷ���ʽ��_____________________________________��ȡ�����ϲ������Һ��������ͨ��������CO2���������µõ���Ӧ�ij���, ���йص����ӷ���ʽΪ________________________��
��4������ƽ���һ����ص�������ԭ����ʽ��
_____Na2Cr2O7 + ______SO2 + _______ H2O =" ______" Cr(OH)(H2O)5SO4 + ______ Na2SO4��ÿ����1mol Cr(OH)(H2O)5SO4ʱ���÷�Ӧ��ת�Ƶĵ�����Ϊ__________��
 CoO2��LiC6����ŵ�ʱ����ص�������ӦʽΪ________________����ͼ��ʾ��װ�ù���ʱ���Ӻ����ӵ��ƶ�����ʱ�õ�ش���_________(��ŵ硱��硱)״̬��
CoO2��LiC6����ŵ�ʱ����ص�������ӦʽΪ________________����ͼ��ʾ��װ�ù���ʱ���Ӻ����ӵ��ƶ�����ʱ�õ�ش���_________(��ŵ硱��硱)״̬��

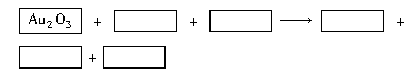
 Ag2O2����2KNO3��2K2SO4��2H2O
Ag2O2����2KNO3��2K2SO4��2H2O