��Ŀ����
 Cu2+����NH3��H2O��OH-��Cl-���γ���λ��Ϊ4������
Cu2+����NH3��H2O��OH-��Cl-���γ���λ��Ϊ4��������1����CuSO4��Һ�м������NaOH��Һ������Na2[Cu��OH��4]��
�ٻ���������[Cu��OH��4]2+�е���λ��
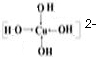

��Na2[Cu��OH��4]�г�����λ���⣬�����ڵĻ�ѧ��������
AC
AC
������ţ���A�����Ӽ� B�������� C�����Թ��ۼ� D���Ǽ��Թ��ۼ�
��2������ͭ�����백ˮ��������ⶼ���ܷ�Ӧ�������백ˮ��������Ļ����Һ�������·�Ӧ��
Cu+H2O2+4NH3�T[Cu��OH��4]2++2OH-����ԭ����
��������Ϊ����������������Cu2+�γ���λ����������ٽ�ʹ��Ӧ����
��������Ϊ����������������Cu2+�γ���λ����������ٽ�ʹ��Ӧ����
����3��Cu2+�������Ҷ�����H2N-CH2CH2-NH2���γ������ӣ���ͼ��
��H��O��N����Ԫ�صĵ縺�ԴӴ�С��˳��
O��N��H
O��N��H
�����Ҷ���������Nԭ�ӳɼ�ʱ��ȡ���ӻ�������
sp3
sp3
�����Ҷ����е����Cl-CH2CH2-Cl����Ҫԭ����
�Ҷ������Ӽ����γ����
�Ҷ������Ӽ����γ����
����������1����Cu2+���пչ����OH-���й¶Ե��ӣ����γ���λ����
��Na2[Cu��OH��4]�г�����λ���⣬���������Ӽ��ͼ��Թ��ۼ���
��2���������������Cu����Cu2+�������백�����γ���λ����
��3���ٸ��ݵ縺�������ڱ��еݱ���ɷ�����
���Ҷ���������Nԭ���γ�4���ļ���
���Ҷ������Ӽ����γ������
��Na2[Cu��OH��4]�г�����λ���⣬���������Ӽ��ͼ��Թ��ۼ���
��2���������������Cu����Cu2+�������백�����γ���λ����
��3���ٸ��ݵ縺�������ڱ��еݱ���ɷ�����
���Ҷ���������Nԭ���γ�4���ļ���
���Ҷ������Ӽ����γ������
����⣺��1����Cu2+���пչ����OH-���й¶Ե��ӣ����γ���λ����������[Cu��OH��4]2+��1��Cu2+��4��OH-�γ���λ�����ɱ�ʾΪ ��
��
�ʴ�Ϊ�� ��
��
��Na2[Cu��OH��4]Ϊ���ӻ�����������Ӽ�������O-HΪ���Թ��ۼ����ʴ�Ϊ��AC��
��2���������������Cu����Cu2+�������백�����γ���λ����
�ʴ�Ϊ����������Ϊ����������������Cu2+�γ���λ����������ٽ�ʹ��Ӧ���У�
��3����ͬ����Ԫ�ش�����Ԫ�صĵ縺������ǿ����O��N��H�ĵ縺������������O��N��H���ʴ�Ϊ��O��N��H��
���Ҷ���������Nԭ���γ�4���ļ���Ϊsp3�ӻ����ʴ�Ϊ��sp3��
��NԪ�ص縺�Խ�ǿ�����γ�������е�ϸߣ��ʴ�Ϊ���Ҷ������Ӽ����γ������
 ��
���ʴ�Ϊ��
 ��
����Na2[Cu��OH��4]Ϊ���ӻ�����������Ӽ�������O-HΪ���Թ��ۼ����ʴ�Ϊ��AC��
��2���������������Cu����Cu2+�������백�����γ���λ����
�ʴ�Ϊ����������Ϊ����������������Cu2+�γ���λ����������ٽ�ʹ��Ӧ���У�
��3����ͬ����Ԫ�ش�����Ԫ�صĵ縺������ǿ����O��N��H�ĵ縺������������O��N��H���ʴ�Ϊ��O��N��H��
���Ҷ���������Nԭ���γ�4���ļ���Ϊsp3�ӻ����ʴ�Ϊ��sp3��
��NԪ�ص縺�Խ�ǿ�����γ�������е�ϸߣ��ʴ�Ϊ���Ҷ������Ӽ����γ������
���������⿼���Ϊ�ۺϣ��漰��λ����Ԫ���������Լ������֪ʶ���Ѷ��еȣ�����ע����λ�����γ�����������Ԫ�����ڵĵݱ���ɣ�Ϊ�������Ҫ���㣬Ҳ���״��㣬ѧϰ��ע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
�����Ŀ