��Ŀ����
����Ŀ��ij�������Ļ�����A������Է�������Ϊ104��̼����������Ϊ92.3%��
(1)A�ķ���ʽΪ____________��
(2)A��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽΪ________________________����Ӧ������____________��
(3)��֪�� ����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ��������Ľṹ��ʽ_______________��
����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ��������Ľṹ��ʽ_______________��
(4)��A��Ϊͬ���칹���һ������Ϊ������(����ͼ)����������Ķ��ȴ�����__________________����

(5)��һ�������£���A�ۺϵõ��ĸ߷��ӻ��������ѧ����ʽΪ_________________________________________����Ӧ����Ϊ_______________
���𰸡� C8H8 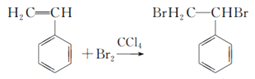 �ӳɷ�Ӧ
�ӳɷ�Ӧ 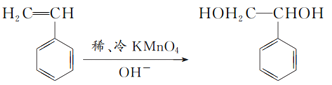 3 ����ʽ�� �Ӿ۷�Ӧ
3 ����ʽ�� �Ӿ۷�Ӧ
��������������Է��������ͺ�̼���ɼ��㺬��������������C��Hԭ����Ŀ����֪����ʽ���������������
��1��1molA��n��C��=104g��92.3%/12g/mol=8mol��n��H��=104g��(192.3%)/1g/mol=8�������ʽΪC8H8����2�����ʺ��б����������巢����Ӧ��˵��AӦΪ����ϩ�����巢���ӳɷ�Ӧ����Ӧ�ķ���ʽΪ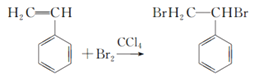 ����3��������֪��Ϣ��֪A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ�ķ���ʽΪ
����3��������֪��Ϣ��֪A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ�ķ���ʽΪ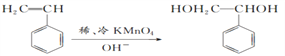 ����������Ľṹ��ʽΪ
����������Ľṹ��ʽΪ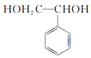 ����4������������Ľṹ��ʽ��֪��������Ķ��ȴ��������֣����������ıߣ���Խ��ߡ���Խ��ߣ���5������̼̼˫�����ɷ����Ӿ۷�Ӧ���ɾ۱���ϩ����
����4������������Ľṹ��ʽ��֪��������Ķ��ȴ��������֣����������ıߣ���Խ��ߡ���Խ��ߣ���5������̼̼˫�����ɷ����Ӿ۷�Ӧ���ɾ۱���ϩ���� ��
��

����Ŀ�����������л��е��������ʣ���ѡ�õ��Լ�������������ȷ���ǣ� ��
���� | ���ʣ������� | �Լ��Ͳ������� | |
A | O2 | H2O�������� | ͨ��ŨH2SO4 |
B | CO2 | CO | ��ȼ |
C | KCl���� | KClO3 | ����MnO2������ |
D | KNO3��Һ | Ba��NO3��2��Һ | ����������Na2SO4��Һ������ |
A.A
B.B
C.C
D.D