��Ŀ����
��8�֣���Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ�ǣ�
CH4(g) + 2O2(g) == CO2(g) + 2H2O��l�� ��H== ��889.6kJ/mol
��ش��������⣺
��1����Ӧ�������ܺ�________������ڡ�����С�ڡ����ڡ��������������ܺ͡�
��2����1 mol������ȫȼ�����ɶ�����̼��ˮ��������ų������� ���������������������889.6kJ��
��3����֪����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ�ǣ�2H2(g)+O2(g) ===2H2O��l�� ��H =��572kJ/mol ������ͬ�����ļ������������ȫȼ������Һ̬ˮ�����Ƚ϶����________��
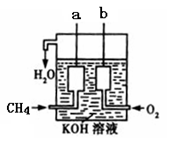
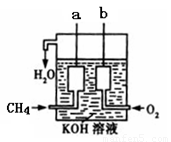
��4����ͼ��ʾ��װ������CH4 ��O2��KOH��Һ��ɵ�����ȼ�ϵ�أ����ø�װ�ÿ��Խ� ��ת��Ϊ �ܡ�
(8��)��1�����ڣ�2�֣� ��2��<��2�֣�
��3��������2�֣� ��4����ѧ�ܣ����ܣ�ÿ�ո�1�֣�
����������1������ȼ���Ƿ��ȷ�Ӧ�����Է�Ӧ�������ܺʹ��������������ܺ͡�
��2��������̬ˮ����������Һ̬ˮ����������˼���ȼ������Һ̬ˮ�ų��������ࡣ������1 mol������ȫȼ�����ɶ�����̼��ˮ��������ų���������889.6kJ��
��3�������Ȼ�ѧ����ʽ��֪����λ�����ļ����������ȫȼ�շų��������ֱ���889.6kJ��16��55.6kJ��572kJ��4��143kJ����������ȼ�շų��������ࡣ
��4��ԭ����ǰѻ�ѧ��ת��Ϊ���ܵ�װ�á�

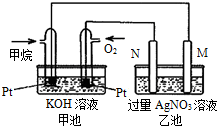 ��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��