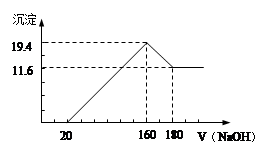��Ŀ����
(9��)
(1)3.6g H2O�����ʵ�����________������________mol H��
(2)�ڱ�״���£�4g H2��11.2 L O2��1mol H2O�У�����������������________������������________�����ѧʽ��
(3)�������Ķ�������������������ǵ����ʵ���֮��Ϊ________����������ԭ����֮��Ϊ________����������ԭ����֮��Ϊ________��
��4���������ʣ���H2O ��ʯī ��NH4NO3 ������ ��CH3COOH ����������������ʵ��� �����ڷǵ���ʵ��� ���ñ����д����
(1)3.6g H2O�����ʵ�����________������________mol H��
(2)�ڱ�״���£�4g H2��11.2 L O2��1mol H2O�У�����������������________������������________�����ѧʽ��
(3)�������Ķ�������������������ǵ����ʵ���֮��Ϊ________����������ԭ����֮��Ϊ________����������ԭ����֮��Ϊ________��
��4���������ʣ���H2O ��ʯī ��NH4NO3 ������ ��CH3COOH ����������������ʵ��� �����ڷǵ���ʵ��� ���ñ����д����
(1)0.2mol��0.4 (2)H2��H2O��(3)5��4��5��4��5��6��4���٢� ��
�����������1��3.6gH2O�����ʵ���Ϊ
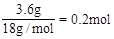 ������0.4molH��
������0.4molH����2����״���£�4gH2�����ʵ���Ϊ
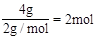 ��11.2LO2�����ʵ���Ϊ
��11.2LO2�����ʵ���Ϊ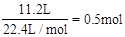 ������Ϊ16g��1molH2O������Ϊ18g����˺�������������H2������������H2O��
������Ϊ16g��1molH2O������Ϊ18g����˺�������������H2������������H2O����3���������Ķ�������������������ǵ����ʵ���֮��Ϊ5��4����������ԭ����֮��Ϊ5��4����������ԭ����֮��Ϊ5��6��
��4���������Ϊ��ˮ�����ᡣ�ǵ����Ϊ�����ǡ�
����������ʺͷǵ���ʶ����ڻ�������ʻ�����Ȳ��ǵ����Ҳ���Ƿǵ���ʡ��������Ҫ��������κͽ���������ǵ������Ҫ�����л���ʹֵķǽ��������ǿ����ʰ���ǿ�ᡢǿ��ʹֵ����࣬������ʰ������ᡢ�����ˮ��

��ϰ��ϵ�д�
�����Ŀ