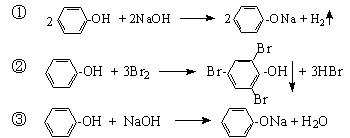
��Ŀ����
��Ӱ������Ϳ�ĸй������Ҫ��AgBr��������ʱ�������ŵ�һ˲�䣬��������Ĺ�ʹAgBr�����˷ֽⷴӦ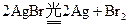 �����ڽ��������٣��ֽ����Ag���٣����Ա���ͨ����ѧ����������Ӱ���ܿ�����Ƭ�ϵ�Ӱ�Ա�������һ�ֳ��õ���Ӱ��������Ӱʱ���������±仯��
�����ڽ��������٣��ֽ����Ag���٣����Ա���ͨ����ѧ����������Ӱ���ܿ�����Ƭ�ϵ�Ӱ�Ա�������һ�ֳ��õ���Ӱ��������Ӱʱ���������±仯��
(1)�ع�ʱ����������Ag����Ӱ������________��
A���������� B���ǻ�ԭ��
C������������� D������ʲô����
(2)��ӰҺ�����ֻ���жԱ����ӣ���Ӱ�ٶ���dz�������Ϊ�����Ӱ�ٶȣ��ɼ���_________��
A��Na2SO4 B��Na2SO3 C��Na2CO3 D��Na2S2O3
(3)������ӰҺ�е���������Ա����ӷ�Ӧ�����������غ�ɫ���۰ߣ�Ӱ���Ƭ��������
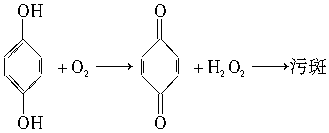
Ϊ�����γ��۰ߣ�������ӰҺ�м���__________��
A��Na2SO4 B��Na2SO3 C��Na2CO3 D��Na2S2O3
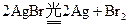 �����ڽ��������٣��ֽ����Ag���٣����Ա���ͨ����ѧ����������Ӱ���ܿ�����Ƭ�ϵ�Ӱ�Ա�������һ�ֳ��õ���Ӱ��������Ӱʱ���������±仯��
�����ڽ��������٣��ֽ����Ag���٣����Ա���ͨ����ѧ����������Ӱ���ܿ�����Ƭ�ϵ�Ӱ�Ա�������һ�ֳ��õ���Ӱ��������Ӱʱ���������±仯��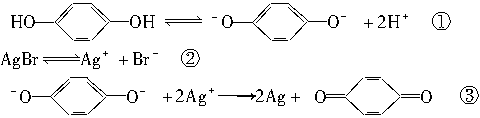
(1)�ع�ʱ����������Ag����Ӱ������________��
A���������� B���ǻ�ԭ��
C������������� D������ʲô����
(2)��ӰҺ�����ֻ���жԱ����ӣ���Ӱ�ٶ���dz�������Ϊ�����Ӱ�ٶȣ��ɼ���_________��
A��Na2SO4 B��Na2SO3 C��Na2CO3 D��Na2S2O3
(3)������ӰҺ�е���������Ա����ӷ�Ӧ�����������غ�ɫ���۰ߣ�Ӱ���Ƭ��������

Ϊ�����γ��۰ߣ�������ӰҺ�м���__________��
A��Na2SO4 B��Na2SO3 C��Na2CO3 D��Na2S2O3
(1)B��(2)C��(3)B
(1)Ag���Ƿ�Ӧ�������������������ԭ���������Ag�������ã���Ӱ��ĵ�Ƭ�ͻ����һƬ���ع�ʱ��������Ag�ܴ���Ӧ�ۣ����Ƭ�������ع�̶Ȳ�ͬ���ֽ����Ag����Ҳ��ͬ�������þ���ǿ��֮����������ʹ��Ƭ�ϳ��ֺڰ����µ�Ӱ��
(2)�ɱ��ӵ������Կ����뵽�Ա�����Ҳ�����ᣬ�Ӽ�(Na2CO3)�ɴ�����룬����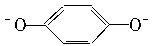 ��Ũ�ȣ������Ӱ�ٶȡ�
��Ũ�ȣ������Ӱ�ٶȡ�
(3)�ɼ��뻹ԭ�ԱȶԱ����Ӹ�ǿ�����ʣ���������ӰҺ�е�O2��Na2SO3��Na2S2O3�䶼�л�ԭ�ԣ�����������AgBr������Ϸ�Ӧ(�Ƕ�Ӱ��)��ӦѡB��
(2)�ɱ��ӵ������Կ����뵽�Ա�����Ҳ�����ᣬ�Ӽ�(Na2CO3)�ɴ�����룬����
 ��Ũ�ȣ������Ӱ�ٶȡ�
��Ũ�ȣ������Ӱ�ٶȡ�(3)�ɼ��뻹ԭ�ԱȶԱ����Ӹ�ǿ�����ʣ���������ӰҺ�е�O2��Na2SO3��Na2S2O3�䶼�л�ԭ�ԣ�����������AgBr������Ϸ�Ӧ(�Ƕ�Ӱ��)��ӦѡB��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
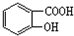
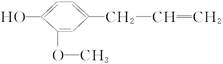 �����й�����������˵����ȷ����( )
�����й�����������˵����ȷ����( )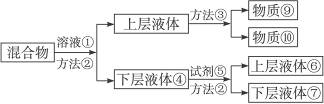
 �������ӣ�
�������ӣ�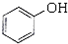 ������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH����
������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH����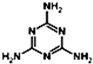 ������Ԥ�������谷�ں˴Ź�������ͼ��1H�˴Ź�����ͼ���л����______���壻�����谷�е�Ԫ�ص���������Ϊ______������ðٷ�����ʾ��������һλС���������������谷��ʹʳƷ�е����ʵĺ�����ߣ�
������Ԥ�������谷�ں˴Ź�������ͼ��1H�˴Ź�����ͼ���л����______���壻�����谷�е�Ԫ�ص���������Ϊ______������ðٷ�����ʾ��������һλС���������������谷��ʹʳƷ�е����ʵĺ�����ߣ�
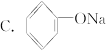 D.Na2SO3
D.Na2SO3