��Ŀ����
��1������5���л������ˮ���ᣨ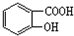 �������ӣ�
�������ӣ�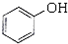 ������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH����
������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH����
����������Na2CO3��Һ��Ӧ����������FeCl3��Һ������ɫ��Ӧ����______��
����������FeCl3��Һ������ɫ��Ӧ����������NaHCO3��Һ��Ӧ����______��
��д����ȩ��������Һ����������Ӧ�Ļ�ѧ����ʽ______��
��д��ˮ����������Ʒ�Ӧ�Ļ�ѧ����ʽ______��
��2���ٵ�������ά����������Ҫ���ʣ������ɰ�����ͨ��______���Ӷ��ɵ�һ��߷��ӻ����������������к��а������Ȼ���������______�ԣ�
��2008�꣬��¹Ӥ���̷�������Ϊ���������谷�����³���ʳ�ø��̷۵IJ���Ӥ����������ʯ���������谷�Ľṹ��ʽ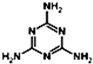 ������Ԥ�������谷�ں˴Ź�������ͼ��1H�˴Ź�����ͼ���л����______���壻�����谷�е�Ԫ�ص���������Ϊ______������ðٷ�����ʾ��������һλС���������������谷��ʹʳƷ�е����ʵĺ�����ߣ�
������Ԥ�������谷�ں˴Ź�������ͼ��1H�˴Ź�����ͼ���л����______���壻�����谷�е�Ԫ�ص���������Ϊ______������ðٷ�����ʾ��������һλС���������������谷��ʹʳƷ�е����ʵĺ�����ߣ�
 �������ӣ�
�������ӣ� ������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH����
������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH��������������Na2CO3��Һ��Ӧ����������FeCl3��Һ������ɫ��Ӧ����______��
����������FeCl3��Һ������ɫ��Ӧ����������NaHCO3��Һ��Ӧ����______��
��д����ȩ��������Һ����������Ӧ�Ļ�ѧ����ʽ______��
��д��ˮ����������Ʒ�Ӧ�Ļ�ѧ����ʽ______��
��2���ٵ�������ά����������Ҫ���ʣ������ɰ�����ͨ��______���Ӷ��ɵ�һ��߷��ӻ����������������к��а������Ȼ���������______�ԣ�
��2008�꣬��¹Ӥ���̷�������Ϊ���������谷�����³���ʳ�ø��̷۵IJ���Ӥ����������ʯ���������谷�Ľṹ��ʽ
 ������Ԥ�������谷�ں˴Ź�������ͼ��1H�˴Ź�����ͼ���л����______���壻�����谷�е�Ԫ�ص���������Ϊ______������ðٷ�����ʾ��������һλС���������������谷��ʹʳƷ�е����ʵĺ�����ߣ�
������Ԥ�������谷�ں˴Ź�������ͼ��1H�˴Ź�����ͼ���л����______���壻�����谷�е�Ԫ�ص���������Ϊ______������ðٷ�����ʾ��������һλС���������������谷��ʹʳƷ�е����ʵĺ�����ߣ�
��1�������ᣨCH3COOH������Na2CO3��Һ��Ӧ����������FeCl3��Һ������ɫ��Ӧ���ʴ�Ϊ�����ᣨCH3COOH����
�ڱ��ӣ�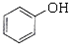 ����FeCl3��Һ������ɫ��Ӧ����������NaHCO3��Һ��Ӧ���ʴ�Ϊ�����ӣ�
����FeCl3��Һ������ɫ��Ӧ����������NaHCO3��Һ��Ӧ���ʴ�Ϊ�����ӣ� ����
����
����ȩ����ȩ��Һ��Ӧ��ԭ������ʽΪ��CH3CHO+2Ag��NH3��2OH
2Ag+3NH3+H2O+CH3COONH4���ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH
2Ag+3NH3+H2O+CH3COONH4��
��ˮ�����еķ��ǻ����Ȼ��������ԣ����Ժͽ����Ʒ�Ӧ��������Ʒ�Ӧ�Ļ�ѧ����ʽΪ��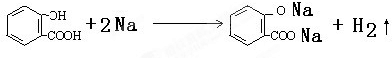 ��
��
�ʴ�Ϊ�� ��
��
��2���ٵ�����ˮ��IJ����ǰ����ᣬ�������ɰ�����ͨ���ļ����Ӷ��ɵ�һ��߷��ӻ�����������м��ԣ��Ȼ��������ԣ���������������ԣ��ʴ�Ϊ���ļ������ԣ�
�ڸ��������谷�Ľṹ��ʽ �ɵ������ʽ��C3H6N6�������е�6����ԭ���ǵ�Ч�ģ��˴Ź�������ͼ��1H�˴Ź�����ͼ�������һ���壬��Ԫ�ص���������Ϊ
�ɵ������ʽ��C3H6N6�������е�6����ԭ���ǵ�Ч�ģ��˴Ź�������ͼ��1H�˴Ź�����ͼ�������һ���壬��Ԫ�ص���������Ϊ
��100%=66.7%���ʴ�Ϊ��1��66.7%��
�ڱ��ӣ�
 ����FeCl3��Һ������ɫ��Ӧ����������NaHCO3��Һ��Ӧ���ʴ�Ϊ�����ӣ�
����FeCl3��Һ������ɫ��Ӧ����������NaHCO3��Һ��Ӧ���ʴ�Ϊ�����ӣ� ����
��������ȩ����ȩ��Һ��Ӧ��ԭ������ʽΪ��CH3CHO+2Ag��NH3��2OH
| �� |
| �� |
��ˮ�����еķ��ǻ����Ȼ��������ԣ����Ժͽ����Ʒ�Ӧ��������Ʒ�Ӧ�Ļ�ѧ����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����2���ٵ�����ˮ��IJ����ǰ����ᣬ�������ɰ�����ͨ���ļ����Ӷ��ɵ�һ��߷��ӻ�����������м��ԣ��Ȼ��������ԣ���������������ԣ��ʴ�Ϊ���ļ������ԣ�
�ڸ��������谷�Ľṹ��ʽ
 �ɵ������ʽ��C3H6N6�������е�6����ԭ���ǵ�Ч�ģ��˴Ź�������ͼ��1H�˴Ź�����ͼ�������һ���壬��Ԫ�ص���������Ϊ
�ɵ������ʽ��C3H6N6�������е�6����ԭ���ǵ�Ч�ģ��˴Ź�������ͼ��1H�˴Ź�����ͼ�������һ���壬��Ԫ�ص���������Ϊ| 14��6 |
| 12��3+1��6+14��6 |

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
�����Ŀ
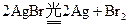 �����ڽ��������٣��ֽ����Ag���٣����Ա���ͨ����ѧ����������Ӱ���ܿ�����Ƭ�ϵ�Ӱ�Ա�������һ�ֳ��õ���Ӱ��������Ӱʱ���������±仯��
�����ڽ��������٣��ֽ����Ag���٣����Ա���ͨ����ѧ����������Ӱ���ܿ�����Ƭ�ϵ�Ӱ�Ա�������һ�ֳ��õ���Ӱ��������Ӱʱ���������±仯��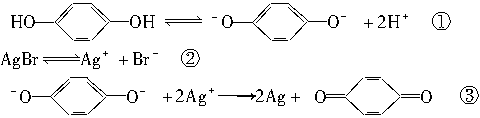
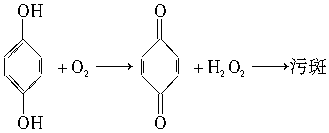
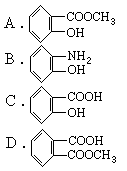
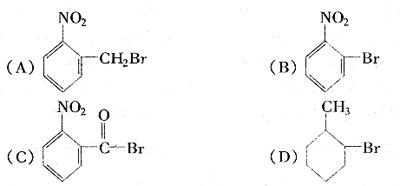
 RCH(OH)SO3Na���ȱ�������ȩ��ˮ�е��ܽ�Ⱥ�С�����ȱ��뱽��ȩ��Һ̬��������ķ����ǣ���NaHSO3��Һ����Һ�����ȱ����ټ�A���ʡ���Һ���ñ���ȩ����A���ʿ�����
RCH(OH)SO3Na���ȱ�������ȩ��ˮ�е��ܽ�Ⱥ�С�����ȱ��뱽��ȩ��Һ̬��������ķ����ǣ���NaHSO3��Һ����Һ�����ȱ����ټ�A���ʡ���Һ���ñ���ȩ����A���ʿ�����