��Ŀ����
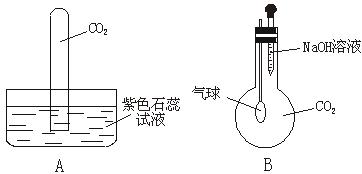
 ij��ѧ��ȤС������ͼ��ʾװ�ý���̽��ʵ�飬����֤����������ϩ��������ϩ���в������ԡ����¶�Ѹ��������170��ɹ۲쵽�Թ�����ˮ��ɫ����ƿ��ŨH2SO4���Ҵ��Ļ��Һ��Ϊ�غ�ɫ��
ij��ѧ��ȤС������ͼ��ʾװ�ý���̽��ʵ�飬����֤����������ϩ��������ϩ���в������ԡ����¶�Ѹ��������170��ɹ۲쵽�Թ�����ˮ��ɫ����ƿ��ŨH2SO4���Ҵ��Ļ��Һ��Ϊ�غ�ɫ��
��1����ͬѧ��Ϊ:���ǵ��û��Һ��Ӧ�ĸ�����,��ˮ��ɫ��������֤����Ӧ������ϩ��������ϩ���в�������,��������ȷ����___________��
A.��ϩ����ˮ����ȡ����Ӧ��
B.ʹ��ˮ��ɫ�ķ�Ӧ��δ���Ǽӳɷ�Ӧ��
C.ʹ��ˮ��ɫ�����ʣ�δ������ϩ��
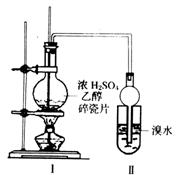
 ��2����ͬѧ����ϸ�¹۲����Ϊ���Թ�����һ�����֤����Ӧ������ϩ���ɣ����������____________________________________________��Ϊȷ����һ��Ӧ�Ǽӳɷ�Ӧ����ȡ����Ӧ������pH��ֽ�����Է�Ӧ�����Һ��������_________________________________________________________________________________________________��
��2����ͬѧ����ϸ�¹۲����Ϊ���Թ�����һ�����֤����Ӧ������ϩ���ɣ����������____________________________________________��Ϊȷ����һ��Ӧ�Ǽӳɷ�Ӧ����ȡ����Ӧ������pH��ֽ�����Է�Ӧ�����Һ��������_________________________________________________________________________________________________��
��3����ͬѧ������ʵ��װ�ý����˸Ľ�����I��II֮��������ͼװ�ã���A�е��Լ�ӦΪ_______________��B�е��Լ�Ϊ_______________��
(1) B��C
(2)Һ��ֲ㣬�²�Ϊ��״Һ�塣
������ȡ����Ӧ������HBr��ˮ��ҺpH���Լ�С���������ӳɷ�Ӧ��ˮ��ҺpHֵ������
(3)NaOH��Һ��������ҺҲ���ԣ�������ʯ��ˮ���ף���1�֣���Ʒ����Һ

 ��У����ϵ�д�
��У����ϵ�д�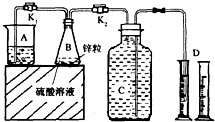 ij��ѧ��ȤС������ͼװ�ý��С�����п�������ᷴӦ��ȡ����������ѡ��̽������B�еĻ�ѧ��Ӧ����ƽ�Ⱥ�ÿ���һ�����ռ�һ����������ͬ�����£�ÿ�����ռ���������������±���
ij��ѧ��ȤС������ͼװ�ý��С�����п�������ᷴӦ��ȡ����������ѡ��̽������B�еĻ�ѧ��Ӧ����ƽ�Ⱥ�ÿ���һ�����ռ�һ����������ͬ�����£�ÿ�����ռ���������������±���| ʵ����� | ����Ũ�� | ��������ͭ��Һ���/mL | ��Ӧ�ﵽƽ�ȵ�ʱ��/min | ��1�����ռ��������/mL | ��2�����ռ��������/mL | ��3�����ռ��������/mL | �� |
| 1 | 20% | 0 | 5.4 | 6.1 | 11.9 | 13.7 | �� |
| 2 | 20% | 0.5 | 2.5 | 23.9 | 32.1 | 33.5 | �� |
| 3 | 30% | 0.5 | 1.1 | 60.7 | 89.9 | 90.1 | �� |
| 4 | a% | 3.0 | 0.8 | 55.7 | 66.0 | 61.4 | �� |
��1���ر�K2����K1���۲쵽
��2��ʵ��l��2�ǶԱ�̽��ʵ�飬�����ϱ���˵���öԱ�̽��ʵ���Ŀ����
��3��ϸ��ƿC���ݻ�ΪV��mL����Ϊ��ʹ�����п������bg�����˷ѣ���C�л�����
| 2 |
| 5 |
��4��ʵ��4���о���������ͭ��Һ�������ʵ���Ӱ�죬aֵӦΪ
A�� 20 B��30 C��27 D�� 40
��5�������ϱ�������п�������ᷴӦ��ȡ����������Ӧ��ѡ��ʵ��
��6����ʵ���ķ�Һ�л���𩷯��ZnSO4?7H2O�������ʵ�����������
��7�������ϵ�֪��Cu++Cl-�TCuCl����
ʪ��ұп�����У�����п��Һ�г����������������ӣ������м�����ͭ��Һ�ͽ���п���ɳ�ȥ�����ӣ������ӷ�Ӧ����ʽ��
 ij��ѧ��ȤС������ͼ��ʾװ�õ��CuSO4��Һ���ⶨͭ����Է���������
ij��ѧ��ȤС������ͼ��ʾװ�õ��CuSO4��Һ���ⶨͭ����Է���������