��Ŀ����
��֪�к��ȵ���ֵ��57.3 kJ��mol�C1���������ʷ�Ӧʱ������57.3 kJ��������
| A��ϡHCl��ϡNaOH |
| B��1.0 mol��L-1 HCl��1.0 mol��L �C1 NaOH |
| C��500 mL 2.0 mol��L-1 HCl��500 mL2.0 mol��L �C1 NaOH |
| D��500 mL 2.0 mol��L-1 H2SO4��500 mL 2.0 mol��L-1 Ba(OH)2 |
C
��������������к�������һ�������£�ϡ��Һ�У�ǿ���ǿ�Ӧ����1molˮʱ���ų���������A��B����ȷ������ˮ�����ʵ���������ȷ��C��ǡ������1molˮ����ȷ��D������2mol��ˮ���ų���������57.3 kJ��2��D����ȷ����ѡC��
���㣺�����к��ȵ��йؼ������ж�
�������������е��Ѷȵ����⣬���������ǿ�����ض�ѧ������֪ʶ�Ĺ�����ѵ����ͬʱ�������������������Ĺؼ�����ȷ�к��ȵĺ��壬Ȼ��������������ü��ɡ�

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�|
��֪�к��ȵ���ֵ��57.3 kJ/mol�����з�Ӧ����ʱ����������������57.3 kJ���� | |
A�� |
1 mol/LϡHCl��1 mol/LϡNaOH |
B�� |
0.5 L��2.0 mol/L��HCl��0.5 L��2.0 mol/L��NaOH |
C�� |
0.5 L��2.0 mol/L�����0.5 L��2.0 mol/L��NaOH |
D�� |
1 mol/LϡCH3COOH��1 mol/LϡNaOH |
ijʵ��С�������50 mL1.0mol/L�����50mL1.1mol/L����������Һ�����кͷ�Ӧ���ⶨ�к��ȣ��к��Ȳⶨʵ��Ĺؼ���Ҫ�Ƚ�ȷ������һ�����ʵ���Ũ�ȵ���Һ��������Ҫ�����������ȣ������ȵĹ�����Ҫ��������������ɢʧ���Ƚ�ȷ�ز�������Ӧǰ����Һ�¶ȵı仯���ش��������⣺
��1���к��ȵIJⶨ����IJ��������� ��
��2����ʵ�������Թ�����NaOH��ԭ���� ��
��3���ڷ�Ӧ������Ϊ�з��������������HCl�ӷ������õ��к�����ֵ ����ƫ�ߡ�ƫ�ͻ䣩��
��4����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50mL������¼����ԭʼ���ݡ�
| ʵ����� w. | ��ʼ�¶�t1/�� w.w.^w.k.&s.5*u.c.#om | ��ֹ�¶ȣ�t2����[��Դ:ѧ����ZXXK][��Դ:ѧ���ơ���] | �²t2-t1����[��Դ:Z+xx+k.Com] | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 w.w.^w.k.&s.5*u.c.#om | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 w.w.^w.k.&s.5*u.c.#om | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 w.w.^w.k.&s.5*u.c.#om | 31.9 w.w.^w.k.&s.5*u.c.#om | 6.8 |
��֪���ᡢNaOH��Һ�ܶȽ���Ϊ1.00g/cm3���кͺ���Һ�ı����� C=4.18J/(g����)��÷�Ӧ���к���Ϊ��H=_______________________________��
��5�����õ�Ũ�ȵĴ������������NaOH��Һ��Ӧ�����õ��к��ȵ���ֵ�� ����ƫ�ߡ�ƫ�ͻ䣩����ԭ�� ![]() ��
��
��֪ϡ������ϡ�ļ���кͷ�Ӧ������1molˮ���ų����Ƚ��к��ȣ���50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺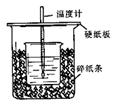
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�� ��
��2�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)�������к��� (���ȡ�����ȡ�)��
��3����֪�����ѧ�߶�һ��ijͬѧ��ʵ���¼����,
| ʵ����� | ����¶� | ����¶� | ��Ӧ����¶� |
| 1 | 21.5�� | 20.5�� | 24.3�� |
| 2 | 21.5�� | 21.5�� | 25.0�� |
| 3 | 16.0�� | 18.5�� | 21.5�� |
��4������ͬŨ�Ⱥ�����İ�ˮ(NH3��H2O)����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� �����ƫ����ƫС��������Ӱ�족����
��֪ϡ������ϡ�ļ���кͷ�Ӧ������1molˮ���ų����Ƚ��к��ȣ���50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
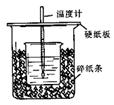
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�� ��
��2�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)�������к��� (���ȡ�����ȡ�)��
��3����֪�����ѧ�߶�һ��ijͬѧ��ʵ���¼����,
|
ʵ����� |
����¶� |
����¶� |
��Ӧ����¶� |
|
1 |
21.5�� |
20.5�� |
24.3�� |
|
2 |
21.5�� |
21.5�� |
25.0�� |
|
3 |
16.0�� |
18.5�� |
21.5�� |
������������ݼ����к��ȣ������ܶȾ���Ϊ1g/ml, C=4.17J/g.�棩
��4������ͬŨ�Ⱥ�����İ�ˮ(NH3��H2O)����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� �����ƫ����ƫС��������Ӱ�족����
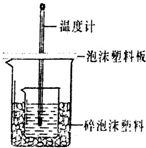 ijʵ��С�������0.55mol/L��NaOH��Һ50mL��0.50mol/L������50mL������ͼ��ʾ��װ���н��вⶨ�к��ȵ�ʵ�飮
ijʵ��С�������0.55mol/L��NaOH��Һ50mL��0.50mol/L������50mL������ͼ��ʾ��װ���н��вⶨ�к��ȵ�ʵ�飮