��Ŀ����
��֪ϡ������ϡ�ļ���кͷ�Ӧ������1molˮ���ų����Ƚ��к��ȣ���50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺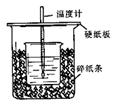
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�� ��
��2�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)�������к��� (���ȡ�����ȡ�)��
��3����֪�����ѧ�߶�һ��ijͬѧ��ʵ���¼����,
| ʵ����� | ����¶� | ����¶� | ��Ӧ����¶� |
| 1 | 21.5�� | 20.5�� | 24.3�� |
| 2 | 21.5�� | 21.5�� | 25.0�� |
| 3 | 16.0�� | 18.5�� | 21.5�� |
��4������ͬŨ�Ⱥ�����İ�ˮ(NH3��H2O)����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� �����ƫ����ƫС��������Ӱ�족����
��1�����ν����� ��2������ȣ���ȣ�3����H=" -56.7KJ/mol" ��4�� ƫС
���������������1���ڷ�Ӧ������Ϊ��ʹ��Һ��Ͼ��ȣ���Ҫ���裬��˴�ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�ǻ��ν�������
��2���ı��������������Ӧ��ʵ�ʷų��������Dz�ͬ�����к����Dz���ġ�
��3�����ݱ������ݿ�֪��1��2��3ʵ�����¶Ȳ�ֵ�ֱ���24.3�棭��21.5�棫20.5�棩��2��3.3�桢25.0�棭��21.5�棫21.5�棩��2��3.5�桢21.5�棭��16�棫18.5�棩��2��4.25��.��Ȼ����ʵ���������ȥ�����¶ȱ仯��ƽ��ֵ��3.4�档��Ӧ������ˮ�����ʵ�����0.025mol�����Ը÷�Ӧ���к����ǡ�H�� ��
��
��4������һˮ�ϰ���������ʣ����ڵ���ƽ�⣬���������ȵģ���������ͬŨ�Ⱥ�����İ�ˮ(NH3��H2O)����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵƫС��
���㣺�����к��ȵIJⶨ���й�ʵ���жϡ������������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ����Ҫ�ǿ���ѧ�����к��Ȳⶨ���˽����ճ̶ȣ�����������ѧ���淶�Ͻ���ʵ��������������ѧ����Ӧ��������ѧϰЧ�ʡ�����������Ҫ����ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���֪ϡ������ϡ�ļ���кͷ�Ӧ������1molˮ���ų����Ƚ��к��ȣ���50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
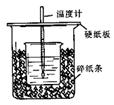
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�� ��
��2�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)�������к��� (���ȡ�����ȡ�)��
��3����֪�����ѧ�߶�һ��ijͬѧ��ʵ���¼����,
|
ʵ����� |
����¶� |
����¶� |
��Ӧ����¶� |
|
1 |
21.5�� |
20.5�� |
24.3�� |
|
2 |
21.5�� |
21.5�� |
25.0�� |
|
3 |
16.0�� |
18.5�� |
21.5�� |
������������ݼ����к��ȣ������ܶȾ���Ϊ1g/ml, C=4.17J/g.�棩
��4������ͬŨ�Ⱥ�����İ�ˮ(NH3��H2O)����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� �����ƫ����ƫС��������Ӱ�족����
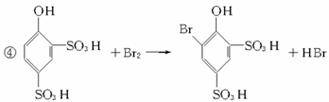
 ��д���йظ�����Ӧ�Ļ�ѧ����ʽ��
��д���йظ�����Ӧ�Ļ�ѧ����ʽ��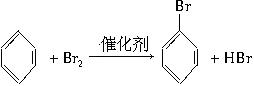 ��ϡ���п�ˮ�⣺
��ϡ���п�ˮ�⣺ 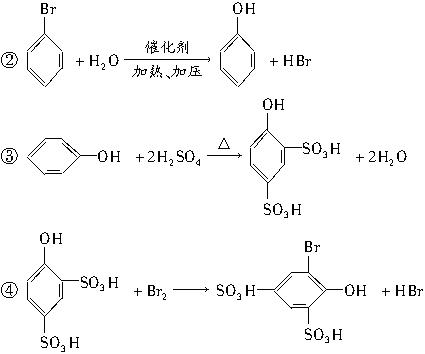

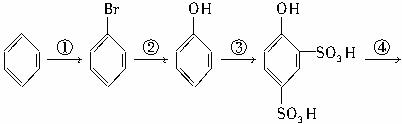 ����д��������Ӧ�ķ���ʽ��
����д��������Ӧ�ķ���ʽ��

 ��д���йظ�����Ӧ�Ļ�ѧ����ʽ��
��д���йظ�����Ӧ�Ļ�ѧ����ʽ��