��Ŀ����
����Ŀ��ij��ѧ��ȤС����̽���������ʵ����ʼ��Ʊ���
��̽��һ��ѡ�������װ�ú�ҩƷ̽��������������������ǿ����
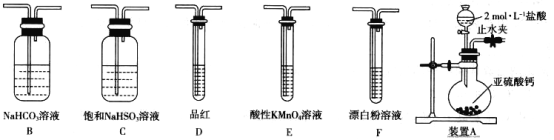
��1��װ��A��ʢ����IJ�������������____________��װ��A�з�Ӧ�����ӻ�ѧ����ʽΪ_______________________________��
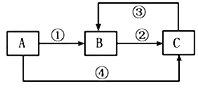
��2��װ������˳��ΪA��________________������װ��C��������_________��ͨ������___________________________������֤�������������ǿ�ڴ����ᡣ
��̽��������������ƾ��壨Na2S2O3��5H2O,M=248g/mol����������Ӱ������ԭ�����ش��������⣺
��3������K2Cr2O7����Һ�����ⶨ��������ƵĴ��ȡ��ⶨ�������£�
����Һ���ƣ���ȡ1.2000 gij��������ƾ�����Ʒ��������в���ȴ������ˮ��__________���ܽ⣬��ȫ�ܽ��ȫ��ת����100mL_________�У��ٶ�������Һ����̶�����ƽ��
�ڵζ���ȡ0.00950 mol��L1��K2Cr2O7����Һ20.00 mL�������ữ��������KI��������Ӧ�� Cr2O72+6I+14H+![]() 3I2+2Cr3++7H2O��Ȼ���������������Ʒ��Һ�ζ���������ɫ��������Ӧ��I2+2S2O32
3I2+2Cr3++7H2O��Ȼ���������������Ʒ��Һ�ζ���������ɫ��������Ӧ��I2+2S2O32![]() S4O62+2I�����������Һ��Ϊָʾ���������ζ�����_______________��Ϊ�յ㡣ƽ�еζ�3�Σ���Ʒ��Һ��ƽ������Ϊ24.80 mL������Ʒ����Ϊ_________%������1λС������
S4O62+2I�����������Һ��Ϊָʾ���������ζ�����_______________��Ϊ�յ㡣ƽ�еζ�3�Σ���Ʒ��Һ��ƽ������Ϊ24.80 mL������Ʒ����Ϊ_________%������1λС������
��4��Na2S2O3���������ȼ�������Һ���ױ���������ΪSO42-���÷�Ӧ�����ӷ���ʽΪ____________________________________��
���𰸡�Բ����ƿ CaSO3+2H+=Ca2++SO2��+H2O CBEDF ��ȥHCl���� װ��D��Ʒ����Һ����ɫ��F�г��ְ�ɫ���� �ձ� ����ƿ �������һ�������������Һ����Һ��ɫ��ȥ���Ұ���Ӻ�ԭ 95.0 S2O32-+4Cl2+5H2O�T2SO42-+8Cl-+10H+
��������
��1��װ��A��ʢ��������Ƶ���������ΪԲ����ƿ��Բ����ƿ�з����ķ�ӦΪCaSO3�����ᷴӦ�Ʊ�SO2��
��2��HClO����ǿ�����ԣ�SO2���л�ԭ�ԣ����߿ɷ���������ԭ��Ӧ����˲�������SO2����������Һ��Ӧ�Ƚ�HClO��H2SO3������ǿ��������֤��H2SO3������ǿ��H2CO3���ٽ��H2CO3������ǿ��HClO���бȽϡ�Aװ�������Ʊ�SO2�����������ӷ���Aװ���Ʊ��������л���HCl���壬ѡ�ñ���NaHSO3��Һ��ȥHCl����ͨ��NaHCO3��Һ��������֤H2SO3������ǿ��H2CO3��������KMnO4��Һ��ȥCO2�е�SO2����Ʒ����Һ����CO2�е�SO2����������ͨ��Ư����Һ�У�������ɫ����������֤��H2CO3������ǿ��HClO���ݴ˻ش�������⡣
��3��������һ�����ʵ���Ũ�ȵ���Һ�IJ���������з�����
�ڵ��������������Һ����ɫ�����������������Һ��Ϊָʾ���������ζ����ⲻ�ϱ����������������һ�������������Һ����Һ��ɫ��ȥ���Ұ���Ӻ�ԭ����Ϊ�յ㣻����Cr2O72+6I+14H+![]() 3I2+2Cr3++7H2O��I2+2S2O32
3I2+2Cr3++7H2O��I2+2S2O32![]() S4O62+2I��֪��Cr2O72--3I2--6S2O32�������n(S2O32),�Ӷ��������m(Na2S2O3��5H2O)�������Ʒ������
S4O62+2I��֪��Cr2O72--3I2--6S2O32�������n(S2O32),�Ӷ��������m(Na2S2O3��5H2O)�������Ʒ������
��4��Na2S2O3����������ΪSO42-����������ԭΪCl-���ݴ�д����Ӧ�����ӷ���ʽ��
��1��װ��A��ʢ��������Ƶ���������ΪԲ����ƿ��Բ����ƿ�з����ķ�ӦΪCaSO3�����ᷴӦ�Ʊ�SO2����ѧ����ʽΪCaSO3+2H+=Ca2++SO2��+H2O������������������ǣ�Բ����ƿ��CaSO3+2H+=Ca2++SO2��+H2O��
��2��HClO����ǿ�����ԣ�SO2���л�ԭ�ԣ����߿ɷ���������ԭ��Ӧ����˲�������SO2����������Һ��Ӧ�Ƚ�HClO��H2SO3������ǿ��������֤��H2SO3������ǿ��H2CO3���ٽ��H2CO3������ǿ��HClO���бȽϡ�Aװ�������Ʊ�SO2�����������ӷ���Aװ���Ʊ��������л���HCl���壬ѡ�ñ���NaHSO3��Һ��ȥHCl����ͨ��NaHCO3��Һ��������֤H2SO3������ǿ��H2CO3��������KMnO4��Һ��ȥCO2�е�SO2����Ʒ����Һ����CO2�е�SO2����������ͨ��Ư����Һ�У�������ɫ����������֤��H2CO3������ǿ��HClO����ˣ�װ�õ�����˳��ΪACBEDF��������Ϸ�����֪��װ������װ��C�������dz�ȥHCl���壻���ݷ�����֪֤�������������ǿ�ڴ����������ΪD��Ʒ����Һ����ɫ��F�г��ְ�ɫ����������������������ǣ�CBEDF�� ��ȥHCl���壻װ��D��Ʒ����Һ����ɫ��F�г��ְ�ɫ������
��3��������һ�����ʵ���Ũ�ȵ���Һ����ȡ����������ƾ�����Ʒ��������в���ȴ������ˮ���ձ����ܽ⣬��������ֽ��裬��ȫ�ܽ��ȫ��ת����100mL����ƿ�У��ٶ�������Һ����̶�����ƽ������������������ǣ��ձ�������ƿ��
�ڵ��������������Һ����ɫ�����������������Һ��Ϊָʾ���������ζ����ⲻ�ϱ����������������һ�������������Һ����Һ��ɫ��ȥ���Ұ���Ӻ�ԭ����Ϊ�յ㣻����Cr2O72+6I+14H+![]() 3I2+2Cr3++7H2O��I2+2S2O32
3I2+2Cr3++7H2O��I2+2S2O32![]() S4O62+2I��֪��Cr2O72--3I2--6S2O32������n(K2Cr2O7)= 0.00950��20.00��10-3mol,��n(Na2S2O3)= 6��0.00950��20.00��10-3mol,m(Na2S2O3)=6��0.00950��20.00��10-3��248g,��������Ʒm(Na2S2O3��5H2O)����Ϊ[6��0.00950��20.00��10-3��248����100/24.8��]/1.2000��100%=95.0%��
S4O62+2I��֪��Cr2O72--3I2--6S2O32������n(K2Cr2O7)= 0.00950��20.00��10-3mol,��n(Na2S2O3)= 6��0.00950��20.00��10-3mol,m(Na2S2O3)=6��0.00950��20.00��10-3��248g,��������Ʒm(Na2S2O3��5H2O)����Ϊ[6��0.00950��20.00��10-3��248����100/24.8��]/1.2000��100%=95.0%��
��������������������������һ�������������Һ����Һ��ɫ��ȥ���Ұ���Ӻ�ԭ ��95.0��
��4��Na2S2O3���������ȼ�������Һ���ױ���������ΪSO42-����������ԭΪCl-���÷�Ӧ�����ӷ���ʽΪS2O32-+4Cl2+5H2O�T2SO42-+8Cl-+10H+���������������������S2O32-+4Cl2+5H2O�T2SO42-+8Cl-+10H+��

 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�����Ŀ����ȥ��Ӧ���л��ϳ������벻���ͼ��ij���;������±�����ʹ�������һ���ʡ�
��1��һ�������²���±������±�������ˮ����Ӧ���P��������±���
±������ | ��Ӧ���P����� |
|
81% 19% |
|
80% 20% |
|
80% 20% |
|
90% 10% |
�����������ݣ��õ�±�����ʹ�������ȥ��Ӧʱ����λ���������Ҫ������_________��
��2���о�һ�����ܷ�����ȥ��Ӧ�Ĵ���д���ṹ��ʽ��_______________________________��
��3����֪��Ũ�������������ˮ����������˳-2-��ϩ(ռ25%)�ͷ�-2-��ϩ(ռ75%)���֡�д��2-��ϩ��˳ʽ�ṹ��_______________________________��
��4����������2-������ϳ�Ȳ��C�ķ�Ӧ���̣�
![]()
A�Ľṹ��ʽΪ_________________��C�Ľṹ��ʽΪ___________________�������еķ�Ӧ������ȥ��Ӧ����___________(����)��