��Ŀ����
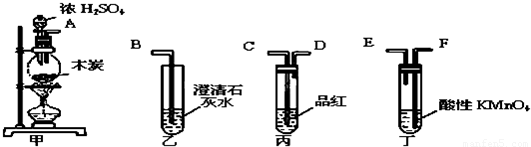
��ѧʵ���У�ͬһ��װ�ÿ������ڲ�ͬ��ʵ�飬����ͼ��ʵ��װ�ã�B�п�Ϊ�����Һ�壬�ɼ��ȣ���
��1����A��ΪŨ���ᣬB��Ϊ������ع��壬D��Ϊʯ����Һ����D������Ϊ________________________��
��2����A��Ϊ������Ũ���ᣬB��Ϊͭ���ʣ�D����NaOH����������������壬��д��D���������ķ�Ӧ�Ļ�ѧ����ʽ________________________________________________��
��3����A��ΪŨH2SO4��B��ΪNaCl���壬 D����Na2S��Na2SO3�Ļ����Һ����Һ©�����ȣ�D�г��ֻ�ɫ�������г�������ζ�������ݳ�����D��n(Na2S)��n(Na2SO3)�������������______________________________��
��4����װ�ÿ���ģ�ⰱ��ƴ��D��Ϊ����ʳ��ˮ������ȡ________����ͨ��D�������ͣ�����ȡ_______����Ҳͨ��D�У���ʱ���Կ�����Һ���о�����������д���÷�Ӧ�Ļ�ѧ����ʽ___________________________________________________��
��5����������װ�ã�����ʵ�鲻����ʵ�ֵ���_____________________
A��֤��̼��ȱ��ӵ�����ǿ B��֤�������������Ա���ǿ
C�����Ҵ��Ʊ���������ϩ D���Ʊ����ռ���������
��6����A��Ϊϡ���ᣬB��Ϊ̼�����ƹ��壬��Һ©����Ӧһ��ʱ�䣬D�����������������������Ҳ��ܽ⣬��D��Һ��ԭ���ʿ�����______________________________��
��1���ȱ�����ɫ��2�֣���
��2��2NO2 +2NaOH��NaNO3 +NaNO2+H2O��2�֣���
��3��n(Na2S) > `2n(Na2SO3)��2�֣���
��4��NH3 CO2 ����1�֣� NH3 + CO2+NaCl + H2O �� NaHCO3�� + NH4Cl��1�֣���
��5��C��2�֣���
��6��NaOH NaAlO2 ���������ɣ���2�֣���
��������
�����������1��Ũ���������ع��巢����Ӧ��������������Ũ�����ӷ����Ȼ��⣬��˳������������������Ȼ���Ļ�����壬������D�е�ʯ����Һʱ��������ˮ��Ӧ�д�����������Ư���ԣ���D������Ϊ�ȱ�����ɫ��
��2��Ũ�����ͭ���ʷ�Ӧ���ɶ����������壬����������NaOH��Ӧ�Ļ�ѧ����ʽ2NO2 +2NaOH��NaNO3 +NaNO2+H2O����3��ŨH2SO4��NaCl���巴Ӧ�����Ȼ������壬 �Ȼ����D����Na2S��Na2SO3�Ļ����Һ��Ӧ�����ɻ�ɫ��������������ζ�����������⣬Ҫ����������ų������ݻ�ѧ����ʽ2H2S +SO2 =3S +2H2O ����D��n(Na2S)��n(Na2SO3)�������������n(Na2S) > `2n(Na2SO3)����4������ƴ��ѡ�ǰѰ���ͨ�뵽ʳ��ˮ�У���ͨ�������̼���壬��Һ���о���̼��������������Ӧ����ʽΪNH3 + CO2+NaCl + H2O �� NaHCO3�� + NH4Cl����5��Ҫ���Ҵ��Ʊ���ϩҪ�õ�Ũ�����������������¶�170�棬��װ����û���¶ȼ����ⶨ��ѡC����6��ϡ�����̼�����ƹ��巴Ӧ���ɶ�����̼���壬D�����������������������Ҳ��ܽ⣬�ݴ˿�֪D��Һ��ԭ���ʿ�����NaOH��NaAlO2 ��
���㣺���⿼��ʵ��װ����ʵ��ԭ����ѡ��



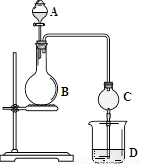
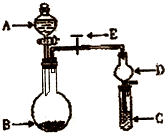 ��2010?����һģ����ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飮��ش�
��2010?����һģ����ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飮��ش� ��ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ��(�г�����ʡ��)����ϵ��ʵ�顣��ش�
��ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ��(�г�����ʡ��)����ϵ��ʵ�顣��ش�