��Ŀ����
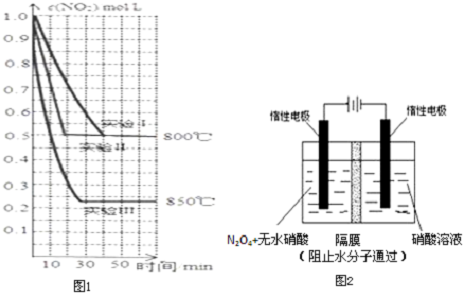
6��CO2��Ϊδ��̼Դ���ȿ��ֲ���ʯ�͡���Ȼ���ȴ�����������ġ�̼ԴΣ�������ֿ���Ч�ؽ������ЧӦ��Ŀǰ���������ù��ܺʹ������ɽ�CO2��H2O��g��ת��ΪCH4��O2��ij�о�С��ѡ�ò�ͬ�Ĵ�����a��b��c������õ�ʵ������ͼ1��ʾ��
��ش��������⣺
��1����Ӧ��ʼ���12Сʱ�ڣ���b����a��b��c���������£��ռ�CH4����࣮
��2��������CH4��H2O��g��ͨ��۽�̫���ܷ�Ӧ����������ӦCH4��g��+H2O��g��?CO��g��+3H2��g����H=+206kJ•mol-1���������ʵ�����CH4��H2O��g������2L�����ܱ�������ij�¶��·�Ӧ5min��ﵽƽ�⣬��ʱ���CO�����ʵ���Ϊ0.10mol����5min��H2��ƽ����Ӧ����Ϊ0.03mol/��L•min����ƽ�����Բ�ȡ����AB�Ĵ�ʩ��ʹn��CO����n��CH4������
A�����������¶�
B�����º�ѹ�³��뺤��
C�����������������
D�����º������ٳ�������ʵ�����CH4��H2O
��3����ҵ�Ͽ�������COΪԭ����ȡCH3OH��
��֪��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.5kJ•mol-1
CO2��g��+H2��g��?CO��g��+H2O��g����H=+41.3kJ•mol-1
����д����CO��H2��ȡ�״����Ȼ�ѧ����ʽCO��g��+2H2��g��?CH3OH��g����H=-90.8kJ•mol-1��
�ڸ÷�Ӧ�ġ�S��0�������������=�������ڵ�������������ڸ÷�Ӧ�Է����У�
��4��ij������ԱΪ�о�H2��CO�ϳ�CH3OH�������ʼ��ɱ�n��H2����n��CO������l L�����ܱ�������ͨ��H2��CO�Ļ������CO��Ͷ������Ϊ1mol�����ֱ���230�桢250���270�����ʵ�飬��ý����ͼ2����230��ʱ��ʵ��������Ӧ��������X������ĸ���������Ǹ÷�Ӧ�Ƿ��ȷ�Ӧ���¶�Խ��ת����Խ�ߣ���ʽ����270��ʱ�÷�Ӧ��ƽ�ⳣ��K��1��
���� ��1��ͼ���������ߣ�b��б��������Է�Ӧ������죻
��2������v=$\frac{��c}{��t}$����v��CO��������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��H2����
A�����������¶ȣ�ƽ�������ƶ���
B�����º�ѹ�³��뺤���������ݻ�����ƽ�������ƶ���
C����������С���������ѹǿ����ƽ�������ƶ���
D�����º������ٳ�������ʵ�����CH4��H2O����ЧΪ��ԭƽ��Ļ���������һ��ѹǿ����ԭƽ����ȣ�ƽ�������ƶ���
��3������֪����CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.5kJ•mol-1
��CO2��g��+H2��g��?CO��g��+H2O��g����H=+41.3kJ•mol-1
���ݸ�˹���ɣ���-��ɵã�CO��g��+2H2��g��?CH3OH��g�����ʱ�Ϊ����֮�
������ӦΪ�������ʵ�����С�ķ�Ӧ����Ϊ�ؼ��ķ�Ӧ����G=��H-T��S��0��Ӧ�Է����У�
��4���ϳɼ״��Ƿ��ȷ�Ӧ�������¶�ƽ�������ƶ���CO��ת���ʼ�С����230��ʱ��ʵ��������Ӧ��������X��270���Ӧ��������Z����������Ϊ2molʱ��COת����Ϊ50%������ƽ��ʱ����ֵ����ʵ��������������Ϊ1L�������ʵ�������Ũ�ȴ���ƽ�ⳣ��K=$\frac{c��C{H}_{3}OH��}{c��CO����{c}^{2}��{H}_{2}��}$���㣮
��� �⣺��1��ͼ���������ߣ�b��б��������Է�Ӧ������죬��ʼ���12Сʱ�ڣ��ռ����ļ�����࣬�ʴ�Ϊ��b��
��2��5min��CO��ƽ����ѧ��Ӧ����v��CO��=$\frac{\frac{0.1mol}{2L}}{5min}$=0.01mol/��L•min��������֮�ȵ��ڻ�ѧ������֮�ȣ�v��H2��=3v��CO��=0.03mol/��L•min����
A�����������¶ȣ���ѧƽ�������ƶ���n��CO������n��CH4����С������֮������A��ȷ��
B�����º�ѹ�³��뺤���������ݻ�����ƽ�������ƶ���n��CO������n��CH4����С������֮������B��ȷ��
C����������С���������ѹǿ����ƽ�������ƶ���n��CO����С��n��CH4��������֮�ȼ�С����C����
D�����º������ٳ�������ʵ�����CH4��H2O����ЧΪ��ԭƽ��Ļ���������һ��ѹǿ����ԭƽ����ȣ�ƽ�������ƶ���n��CO����n��CH4����С����D����
�ʴ�Ϊ��0.03mol/��L•min����AB��
��3������֪����CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.5kJ•mol-1
��CO2��g��+H2��g��?CO��g��+H2O��g����H=+41.3kJ•mol-1
���ݸ�˹���ɣ���-��ɵã�CO��g��+2H2��g��?CH3OH��g����H=-90.8kJ•mol-1
�ʴ�Ϊ��CO��g��+2H2��g��?CH3OH��g����H=-90.8kJ•mol-1��
������ӦΪ�������ʵ�����С�ķ�Ӧ����Ϊ�ؼ��ķ�Ӧ����S��0������ӦΪ���ȷ�Ӧ�����H��0����G=��H-T��S��0��Ӧ�Է����У����������ڷ�Ӧ�Է����У�
�ʴ�Ϊ���������£�
��4���ϳɼ״��Ƿ��ȷ�Ӧ�������¶�ƽ�������ƶ���CO��ת���ʼ�С����230��ʱ��ʵ��������Ӧ��������X��270���Ӧ��������Z����������Ϊ2molʱ��COת����Ϊ50%����
CO��g��+2H2��g��?CH3OH��g��
��ʼ����mol����1 2 0
�仯����mol����0.5 1 0.5
ƽ������mol����0.5 1 0.5
���������Ϊ1L�������ʵ�������Ũ�ȼ��㣬��ƽ�ⳣ��K=$\frac{c��C{H}_{3}OH��}{c��CO����{c}^{2}��{H}_{2}��}$=$\frac{0.5}{0.5��{1}^{2}}$=1��
�ʴ�Ϊ��X���÷�Ӧ�Ƿ��ȷ�Ӧ���¶�Խ��ת����Խ�ߣ�1��
���� ���⿼�黯ѧƽ����㼰Ӱ�����ء�ƽ�ⳣ�������ʼ��㡢�Ȼ�ѧ����ʽ��д�ȣ�ּ�ڿ���ѧ���Ի���֪ʶ���������ã��Ѷ��еȣ�

| HA | H2B | H2C | H3D |
| 1.8��10-5 | K1=4.3��10-7 K2=5.6��10-11 | K1=9.1��10-8 K2=1.1��10-12 | K1=7.5��10-3 K2=6.2��10-8 K3=2.2��10-13 |
��2��HA��H2B��H2C��H3D��������������ǿ����H3D����������H2C��
��3����10mL 0.01mol/L Na2B��Һ����μ���10mL 0.01mol/L HA��Һ������ֽ��裬��Ӧ�����ӷ���ʽ��B2-+HA=HB-+A-��
��4���������ʵ�����HA��H2B��H2C��NaH2D����ˮ��ɻ����Һ������μ����ռ���Һ���������������������ռӦ�����ӷ���ʽ��H3D+OH-=H2O+H2D-���������������ռ���Һ�������OH-��Ӧ��������HD2-+OH-=H2O+D3-��
CH2OH��CHOH��4CHO+Br2+H2O��C6H12O7���������ᣩ+2HBr
2C6H12O7���������ᣩ+CaCO3��Ca��C6H11O7��2����������ƣ�+H2O+CO2��
������ʵ��ܽ��Լ��±���
| �������� | ��������� | �������� | �廯�� | �Ȼ��� |
| ˮ�е��ܽ��� | ��������ˮ��������ˮ | ���� | ���� | ���� |
| �Ҵ��е��ܽ��� | �� | �� | ���� | ���� |
��������Һ�μ�3%��ˮ/55�棺�ٹ���CaCO3/70��ڳ��ȹ��� ���Ҵ� ������Һ
���ˢ�ϴ�Ӣ����Ca��C6H11O7��2
��������Һ$��_{��}^{�μ�3%��ˮ/55��}$ $\underset{\stackrel{����CaC{O}_{3}/70��}{��}}{��}$ $��_{��}^{���ȹ���}$ $��_{��}^{�Ҵ�}$ ����Һ$��_{��}^{����}$ $��_{��}^{ϴ��}$$��_{��}^{����}$Ca��C6H11O7��2
��ش��������⣺
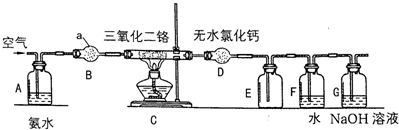
��1���� �ٲ�����ˮ����������ʱ������װ������ʵ���B������ţ���
��2���� �ڲ���ַ�Ӧ��CaCO3��������ʣ�࣬��Ŀ����������������ת���ʣ��ҹ�����̼����׳�ȥ��
��3����ʵ���в�����CaCl2���CaCO3���������Ȼ�����������������ֱ�ӷ�Ӧ�Ƶ���������ƣ�
��4���� �۲�����ȹ��ˣ���ԭ���������������ȴ���ᾧ�������粻���ȹ��˻���ʧ��Ʒ��
��5����������Һ������Cu��OH��2����Һ��Ӧ�Ļ�ѧ����ʽΪCH2OH��CHOH��4CHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$CH2OH��CHOH��4COONa+Cu2O��+3H2O��
| A�� | ��ǿ | B�� | ���� | C�� | ���� | D�� | ���ж� |
| A�� | ���ȶ��ԣ�HCl��HBr��HI | |
| B�� | ���İ뾶��Cl-��Na+��Mg2+ | |
| C�� | ���ӵĻ�ԭ�ԣ�S2-��Cl-��F- | |
| D�� | ����̶ȣ�ͬ�¶�ͬŨ����Һ�У���HCl��CH3COOH��NaHCO3 |