��Ŀ����
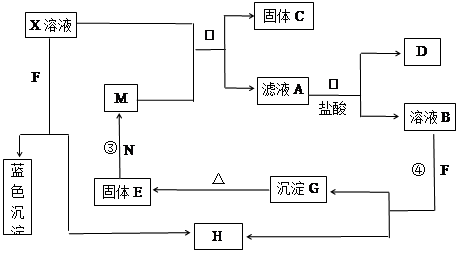
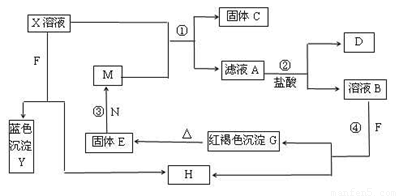
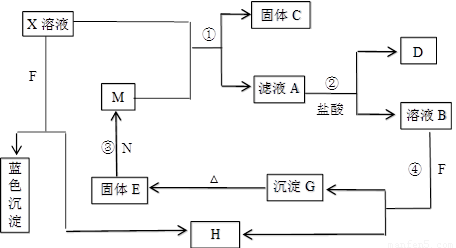
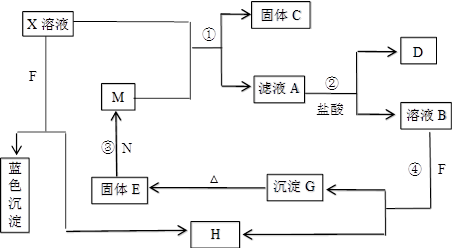
��8�֣���֪XΪ��ѧ��ѧ�е�һ�ֳ������Σ�FΪ����ɫ���壻M��NΪ�����Ľ�����N�����Ԫ�ص����Ӱ뾶�ǵ�������Ԫ�������Ӱ뾶��С�ġ�N��������������ͻ���ϣ����ľ���Ӳ�Ⱥܴ����ֱ�����У�HΪ���嵥�ʣ�DΪ��ɫ���壬�ڿ����л���ֺ���ɫ�������ʵ�ת����ϵ����ͼ�����ַ�Ӧ��������ȥ����
��ش��������⣺
��1��X�Ļ�ѧʽΪ ��F�ĵ���ʽΪ ��
��2����Ӧ�ڵ����ӷ���ʽΪ_______________________________________________��
��Ӧ�۵Ļ�ѧ����ʽΪ_______________________________________________��
��3������100 mL ��X����Һ�м���10 g��������M�ķ�ĩ����ֽ�����ˣ����
��10.16g����C������ҺA�����ʵ����ʵ���Ũ��Ϊ________________________��(�����������)
��1����
��1��/������2�֣�
��2���� 3Fe2+ + NO3��+ 4H+ == 3Fe3+ + NO��+ 2H2O��2�֣�
�� ��2�֣�
��3��0.2 mol/L��2�֣�
����:������������ͼ�⣬˼ά�������ۺ���ǿ���������Ͷ�����ؼ��������ۣ�Ѱ��ͻ�Ƶ㡣���������֪F�ǹ������ƣ�N��Al��D��NO��������������ˮ������������H����������ɫ������������ͭ����X�к���ͭ���ӡ�M��X�ɷ����û���Ӧ���ɹ���C����C��ͭ�����M��������Ӧ������NO��˵����������ԭ��Ӧ������A������������B�к��������ӣ��������Ʒ�Ӧ�����������������������ֽ������������������������������ȷ�Ӧ���õ������ʡ�����ͭ������Ӧ�ķ���ʽΪCu(NO3)2��Fe=Cu��Fe(NO3)2��ÿ����1mol����ͭ��������������8g��ʵ�����ӵ�������0.16g�������ĵ�����ͭΪ![]() ������Ũ����0.2mol/L��
������Ũ����0.2mol/L��