��Ŀ����
(15��)��ѧ����������Ĺؼ�����ѧΪ����������������ṩ�����ʱ�֤��
(1)���ʱ���öƲ������������������ ��Ϊ��ʹ�Ʋ��Ⱦ��ȡ��⻬���ܡ���Ƽ��ĸ�����ǿ����������Һ������Ũ���⣬ͨ�������Բ�ȡ�Ĵ�ʩ��
(2)±ˮ���̺��ŷḻ��þ��Դ����ת����ɻ��MgCl2�ֲ�Ʒ����±ˮ����ȡþ�IJ���Ϊ��
a�������ߴ������ڵı������ճ�ʯ�ң�����ʯ���Ƴ�ʯ���飻
b����ʯ������뵽��ˮ�������о����˵õ�Mg(OH)2������
c.��Mg(OH)2�����м�������õ�MgCl2��Һ���پ������ᾧ�õ�MgCl2��6H2O��
d����MgCl2��6H2O��һ�������¼��ȵõ���ˮMgCl2��
e��������ڵ��Ȼ�þ�ɵõ�Mg��
�ٲ���d�еġ�һ��������ָ���� ��Ŀ���� ��
��������ȡþ��������,Ϊ�˽��ͳɱ���������Ⱦ�����Բ�ȡ�ܶ��ʩ����д������һ��
����ͬѧ��Ϊ������b��ɼ���Mg(0H)2�õ�Mg0���ٵ�����ڵ�MgO�ƽ���þ�������ɼ�ʵ�鲽�裬��ͬ���ͬѧ���뷨��?Ϊʲô?
��
(3)���Ǻ˷�Ӧ����Ҫ��ȼ�ϣ��Ѿ����Ƴɹ�һ���������ӽ�����֬����ר��������ˮ�е�U4+��������������Ԫ�ء��䷴Ӧԭ��Ϊ (��֬��HR����)���������ӽ���������ӽ���Ĥ���ᴦ�������������õ����˵���Һ���䷴Ӧԭ�� Ϊ ��
(4)��˾ƥ��( )�ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ��
���˷�Ӧ����������
��
)�ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ��
���˷�Ӧ����������
��
(1)�Ʋ����������ʧȥ���ӣ���ʱ������Һ�е����ӣ�ʹ��Һ������Ũ�ȱ��ֲ��䣬�Ӷ�ʹ��Ƶ��ٶȱ��ֲ��䣬ʹ�Ʋ��Ⱦ��� (2��)�ʵ����͵��ʱֱ����Դ�ĵ�ѹ���ڵ��Һ�м��������ı� ����Լ�(2��)
(2)����HCl������(1��) ����MgCl2ˮ��(1��) �ڵ�������C12������ȡHCl����(1��)
�۲�ͬ�⣬��ΪMgO�۵�ܸߣ�����ʱ��ķѴ��������������������ɱ�(3��)
(3)4HR+U4+=UR4+4H+(1��) UR4+4H+=4HR+U4+(1��)
(4)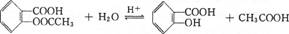 (2��) ˮ�ⷴӦ(��ȡ����Ӧ)(1��)
(2��) ˮ�ⷴӦ(��ȡ����Ӧ)(1��)
����������1������������������ŵ磬���ԶƲ����������ʧȥ���ӣ���ʱ������Һ�е����ӣ�ʹ��Һ������Ũ�ȱ��ֲ��䣬�Ӷ�ʹ��Ƶ��ٶȱ��ֲ��䣬ʹ�Ʋ��Ⱦ��ȡ����ڵ�ѹԽ�����Ի�Ҫ�ʵ����͵��ʱֱ����Դ�ĵ�ѹ���ڵ��Һ�м��������ı�����Լ���
��2���������Ȼ�þˮ�������ԣ����Ȼ�ٽ�ˮ�⣬����Ӧ������HCl�����м��ȣ�������MgCl2ˮ�⡣�������ж������Ա������ѭ�����á����Ե�������C12��������ȡHCl���塣����Ϊ����þ���۵�̫�ߣ�����ʱ��ķѴ��������������������ɱ���
��3���������������������֪��Ӧ�ķ���ʽΪ)4HR+U4+=UR4+4H+�������൱���ǿ��淴Ӧ�����Է���ʽΪUR4+4H+=4HR+U4+��
��4�����ݽṹ��ʽ��֪����˾ƥ���к��������������ܷ���ˮ�ⷴӦ������ʽΪ
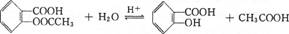 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� (��ѧ�뼼����15��)������������Ÿ��ָ�������;���������﹤��ѧ����������ӿ�ʯ����ȡ������ͭ��һ�ֺ��м�ֵ�Ľ��������Դ�ͭ������ȡ������Dz���ijЩϸ�����ÿ����е�����������ͭ��ʯ���Ѳ����Ե���ͭת���ɿ��ܵ�����ͭ������ϸ����ȡͭ���������̣�������ϸ�����ڵ���ʯ����ʯ���У�������ˮ�Դٽ�ϸ�������������ǵ����������У��������ɵ�����ͭ�γɵ�Ũ�ȵ���Һ������ʯ�ѵĵײ����ٴ�������Һ����ȡ����ͭ��ˮѭ��ʹ�ã��ٻص���ʯ���С���������10%��ͭ�������ַ��������ġ�
�Իش��������⣺
��1��ϸ������ͭ����Ϊ����ͭ�Ĺ���������ʲô���ã�
��2��������ͭ��Һ����ȡͭ���������õķ����� ����Ӧ�Ļ�ѧ����ʽΪ�� ��
��3����ͨ����ͭ�ķ������ڿ�����ȼ����ͭ����������һ����̬��������ԱȽ����ַ�������ȱ�㡣
��4����һ����������������ϡH2SO4��������CuO�Ƴɵ���ͭ����������������ַ�������FeH2Cu����CuOCuSO4Cu
����ʵ��ԭ����в������������Ƶõ���ͭ�����ıȽ��У���ȷ���� (����)
| A���ٶ� | B���ڶ� | C����� | D�����ж� |
��6����ҵ���Ʊ��Ȼ�ͭʱ���ǽ�Ũ����������������80�����ң����������������ͭ��ĩ(��������������)����ֽ��裬ʹ֮�ܽ⣬��Ӧ���£�
CuO��2HCl===CuCl2��H2O FeO��2HCl===FeCl2��H2O
��֪��pH��9.6ʱ��Fe2����Fe(OH)2��ʽ��ȫ������pH��6.4ʱ��Cu2����Cu(OH)2����ʽ�� ȫ������pH��3��4ʱ��Fe3����Fe(OH)3����ʽ��ȫ��������ȥ��Һ�е�Fe2�������Բ��õķ�����
 )�ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ�� ���˷�Ӧ���������� ��
)�ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ�� ���˷�Ӧ���������� ��