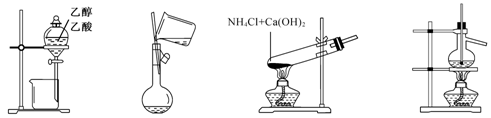
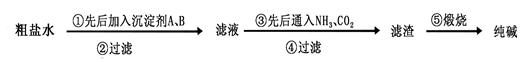
��Ŀ����
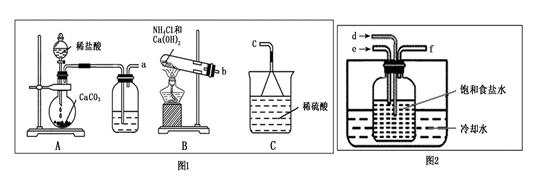
ij�ۺ�ʵ��С��������ˮ���ιۣ��˽Դˮ����������ˮ�Ĺ�������ʾ��ͼ��
��ͼ��ʾ��

�ṩ���Լ�������K2CO3��Һ��NaOH��Һ��Ba(NO3)2��Һ��75�����Ҵ�����ʯ�ҡ�CCl4��BaCl2��Һ
��1��Ϊ��ȥԴˮ�к���Ca2����Mg2����HCO3����Cl����SO42�������ӣ�ѡ��a���������Լ����������˳������Ϊ ��ֻ�ѧʽ����
��2���������ۼ����Գ�ȥ���е���������������ù����� ��ѡ�����и������ţ���
A��ֻ�������仯����ѧ�仯
B��ֻ�л�ѧ�仯���������仯
C�����л�ѧ�仯�����������仯
FeSO4��7H2O�dz��õ����ۼ���������������ɺ��ɫ��״�����������ֽ�״�����Ļ�ѧʽΪ ��
��3��ͨ������CO2��Ŀ���� �� ��
��4�����������У���������������Cl2���� ����д��ţ���
�� ClO2 �� O3 �� Ũ��ˮ �� SO2 �� Ũ����
��ͼ��ʾ��

�ṩ���Լ�������K2CO3��Һ��NaOH��Һ��Ba(NO3)2��Һ��75�����Ҵ�����ʯ�ҡ�CCl4��BaCl2��Һ
��1��Ϊ��ȥԴˮ�к���Ca2����Mg2����HCO3����Cl����SO42�������ӣ�ѡ��a���������Լ����������˳������Ϊ ��ֻ�ѧʽ����
��2���������ۼ����Գ�ȥ���е���������������ù����� ��ѡ�����и������ţ���
A��ֻ�������仯����ѧ�仯
B��ֻ�л�ѧ�仯���������仯
C�����л�ѧ�仯�����������仯
FeSO4��7H2O�dz��õ����ۼ���������������ɺ��ɫ��״�����������ֽ�״�����Ļ�ѧʽΪ ��
��3��ͨ������CO2��Ŀ���� �� ��
��4�����������У���������������Cl2���� ����д��ţ���
�� ClO2 �� O3 �� Ũ��ˮ �� SO2 �� Ũ����
��12�֣�ÿ��2 �֣���1��BaCl2��CaO��2�֣� ��2��C (2�֣���Fe(OH)3��2�֣�
��3����ȥCa2+��������Һ��pH����4�֣� ��4���� �� ��2�֣����������֣�
��3����ȥCa2+��������Һ��pH����4�֣� ��4���� �� ��2�֣����������֣�
�����������1����ʯ������ˮ�����������ƣ��������ƺ�þ���ӽ������������þ��ɫ��������HCO3����Ӧ����CO32������������̼��Ƴ�������SO42���ܺ�Ba2��������ɰ�ɫ�������ᱵ������Ϊ������Ba2����ҪCO32����ȥ�����������˳������ΪBaCl2��CaO��
��2�������������ɾ����������ܵĽ��壬�ù����ǻ�ѧ�仯��ˮ�ľ��������������仯�����Դ�ѡC���������Ӿ��л�ԭ�ԣ��ܱ��������������ӣ����Ժ��ɫ������������������3��ͨ�������̼��������Һ��̼�������Ũ�ȣ���������ӷ�Ӧ���ɳ������Ӷ���ȥ�����ӣ���������Һ�ļ��ԣ�������Һ�����ȡ�
��4����������ǿ�����ԣ���ɱ������������ΪCl2�����Ʒ�����������ǿ�����ԣ�ѡ����ֻ�Т٢ڢܾ���ǿ�����ԣ�������Ũ������и�ʴ�ԣ�������Ϊ����ˮ����������ֻ�Т٢ڷ������⣬��˴�Ϊ�٢ڡ�
���������������ϵ����ʵ�ʣ�������������ǿ�������е��Ѷȵ����⣬�����ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԡ���ȷ����ԭ���������ʵ����ʡ������Ļ�ѧ��Ӧ�ǽ����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ