��Ŀ����
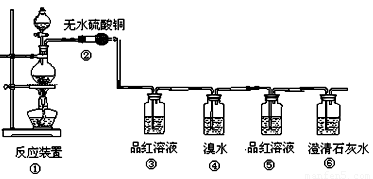
����С������14�֣�ij�о���ѧϰС������֤���ȵ�̿��Ũ���ᷢ����Ӧ�����ɵĸ��ֲ��������������������������ʵ�����̣�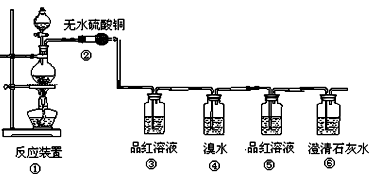
��1�����з�Ӧ�Ļ�ѧ����ʽΪ ���������������� ������ ��
��2���ڴ��������ǣ���������������������������������������������
��3��Ʒ����Һʹ�������Σ���һ��ʹ��ʱ��������������������������������
�ڶ���ʹ��ʱ��������________________ �������� _��
��4����������Ҳ��ʹ��ˮ��ɫ�������˶�������� �� �ԡ�������ԭ������Ư�ס���
��Ӧ�Ļ�ѧ����ʽΪ������������������������������������������
��5������������������������������������������֤��������һ����CO2����
��1��C + 2H2SO4(Ũ)  CO2��+ 2SO2��+ 2H2O
CO2��+ 2SO2��+ 2H2O
��2����ɫ�������ɫ
��3�������Ƿ���SO2���ɣ�������SO2�Ƿ����
��4����ԭ����SO2 + Br2 + 2H2O ="=" H2SO4 + 2HBr
��5������Ʒ����Һ����ɫ�����г���ʯ��ˮ����ǡ�
����

����С������14�֣�a��b��c��d��e���ֶ�����Ԫ�ص�ԭ������������aΪ�ǽ���Ԫ�أ���a��eͬ���壬c��dΪͬ���ڵ�����Ԫ�ء�eԭ�ӵ�����������c��dԭ������������֮�͡�bԭ���������������ڲ��������2����c���⻯���������3�����ۼ������ƶϣ�
��1��д��bԪ�������ڱ��е�λ�� ��
��2����a��c��d���γɵ����ӻ������� ����e������������Ӧˮ�������Һ����ʱ��Ӧ�����ӷ���ʽ�� ��
��3��c�ĵ��ʵĵ���ʽΪ ��
��4��b��d��ȣ��ǽ����Խ�ǿ���� ����Ԫ�ط��ű�ʾ����������ʵ��֤����һ���۵��� ��ѡ����ĸ��ţ���
| A�������£���ĵ��ʳʹ�̬����ĵ��ʳ���̬ |
| B������⻯����ȶ���ǿ�ڣ���⻯�� |
| C��������γɵĻ������У�������� |
| D������⻯��ķе���ڣ���⻯�� |