��Ŀ����
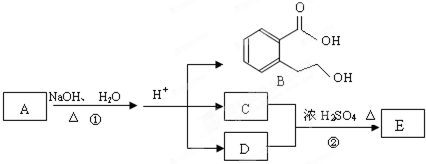
��ͼ�� A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3������Ӧ���úͣĵ���Է���������ȣ���EΪ��֧���Ļ����

������ͼ�ش����⣺
��1��C�����еĹ����������� ______________��������B���������ķ�Ӧ�� ������ĸ��ţ���
a �ӳɷ�Ӧ bȡ����Ӧ c��ȥ��Ӧ d������Ӧ eˮ�ⷴӦ f �û���Ӧ
��2����Ӧ�ڵĻ�ѧ����ʽ��_________________ _��
��3����Ӧ��ʵ���м��ȵ�Ŀ���ǣ�
�� ����
�� ����
��4��A�Ľṹ��ʽ�� __________________ ��
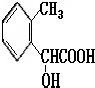
��5��ͬʱ������������������B��ͬ���칹�����Ŀ�� ����
���м��ȡ�������ṹ �����ڷǷ������� ���� FeCl3 ��Һ������ɫ��Ӧ��
д����������һ��ͬ���칹��Ľṹ��ʽ ��
��6�������£���C��Һ��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH
���±���
| ʵ���� | C���ʵ���Ũ�ȣ�mol��L-1�� | NaOH���ʵ���Ũ�ȣ�mol��L-1�� | �����Һ��pH |
| m | 0��1 | 0��1 | pH��9 |
| n | 0��2 | 0��1 | pH��7 |
��m��������������û����Һ����ˮ�������c��OH������ mol��L��1��
n������Һ������Ũ���ɴ�С��˳���� ��
����:����ͼ֪��C��D����������Ӧ����E������֪C�ܸ�NaHCO3������Ӧ���úͣĵ���Է���������ȣ�C�����ᡢD�Ǵ��������к�̼ԭ����С�ڴ���Ҳ��CΪCH3COOH��DΪC3H5OH���پ�E��֧����DΪ1-������
��1��CΪCH3COOH�������Ȼ���������B�в������ṹ�Ŀ���ˮ��Ĺ����š�
��2���ڷ�ӦΪCH3COOH��1-������������Ӧ��ע����дʱע����Ӧ���������桢Ũ���ᡢ���ȡ�
��3����Ӧ��Ϊ������Ӧ���ù��̿��棬����һ�����ܼӿ컯ѧ��Ӧ���ʣ���һ�����ܼ�ʱ�����������������ʹƽ�������ƶ�����߷�Ӧ��IJ��ʡ�
��4������ͼ֪���ٷ�ӦΪˮ�ⷴӦ����B��C��D�������ɵõ�A����
 ��5������Ŀ����֪��ͬ���칹���к��з��ǻ��������λ����һ��ȡ��������ȡ����������ԭ���ţ���C3H5O2�����ɣ��Ҹ�ȡ�����к���������ˮ����Ȼ����ں��������л����У������ǡ�
��5������Ŀ����֪��ͬ���칹���к��з��ǻ��������λ����һ��ȡ��������ȡ����������ԭ���ţ���C3H5O2�����ɣ��Ҹ�ȡ�����к���������ˮ����Ȼ����ں��������л����У������ǡ�
 ��6�� �ɱ���֪��m�������ʵ������Ӧ��ˮ���Լ��ԣ��ʻ����Һ����ˮ�������c��OH����=
��6�� �ɱ���֪��m�������ʵ������Ӧ��ˮ���Լ��ԣ��ʻ����Һ����ˮ�������c��OH����=
![]() =10��5��n���������ʹ�û��Һ�����ԣ� ���� c��CH3COO���� ��c��Na���� ��c��H������ c��OH������
=10��5��n���������ʹ�û��Һ�����ԣ� ���� c��CH3COO���� ��c��Na���� ��c��H������ c��OH������
�𰸣���10�֣���1���Ȼ� �� e
��2��CH3COOH + CH3CH2CH2OH![]() CH3COOCH2CH2CH3+ H2O
CH3COOCH2CH2CH3+ H2O
��3���ټӿ췴Ӧ����
�ڼ�ʱ�������������������������ƽ����������������ķ����ƶ�
��4�� ��
��
��5��4 �� 
д������֮һ����
��6�� 10 -5 c��CH3COO���� ��c��Na���� ��c��H������c��OH����


 ����1��
����1��



 ��ͼ��a��b��c��ʾ��Ӧ�����м�����Լ���������ͼװ����ȡ���������ռ��������ǣ�������
��ͼ��a��b��c��ʾ��Ӧ�����м�����Լ���������ͼװ����ȡ���������ռ��������ǣ�������


 CH2-CH2
CH2-CH2 n��CH2=CH2+H2O
n��CH2=CH2+H2O