��Ŀ����
����Ŀ��A��X��W��D��EΪ������Ԫ�أ���ԭ��������������A��Dͬ���壬X��Wͬ���ڣ� X�γɵ���̬�⻯���ʹʪ��ĺ�ɫʯ����ֽ������ A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȡ�
��1��W��ԭ�ӽṹʾ��ͼΪ________��
��2��A��W����Ԫ�����γɺ�18���ӵķ��ӣ���д���÷��ӵĵ���ʽ��_______________���õ���ʽ��ʾ������D2W���γɹ���______________________��
��3��������ʵ��˵��WԪ�صķǽ����Ա�SԪ�صķǽ�����ǿ����________ (����ĸ)��
a��W������H2S��Һ��Ӧ����Һ�����
b����������ԭ��Ӧ�У�1 mol W���ʱ�1 mol S�õ��Ӷ�
c��W��S��Ԫ�صļ��⻯�����ȷֽ⣬ǰ�ߵķֽ��¶ȸ�
��4��X������������Ӧˮ�����������̬�⻯�����γɵĻ������к��еĻ�ѧ��������_______
��5����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⡣���ڸ���A2W2��ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ________��
���𰸡�

![]() a��c ���Ӽ����ۼ������Լ��� Cu+H2O2+2H+=Cu2+ +2H2O
a��c ���Ӽ����ۼ������Լ��� Cu+H2O2+2H+=Cu2+ +2H2O
��������
A��X��W��D��EΪ������Ԫ�أ���ԭ��������������A��Dͬ���壬X��Wͬ���ڣ� X�γɵ���̬�⻯���ʹʪ��ĺ�ɫʯ����ֽ��������XΪNԪ�أ� A��W���γ�����Һ̬������A2W��A2W2����AΪHԪ�أ�WΪOԪ�أ�DΪNaԪ�أ�EԪ�ص���������������������ȣ���EΪAlԪ�أ�
(1) ����������֪WΪO��O��Ԫ�����ڱ��еĵڶ����� ��VIA�壻O��ԭ�ӽṹʾ��ͼΪ�� ���𰸣�
���𰸣�
(2) AΪH��WΪOԪ�أ�A��W ����Ԫ���γɵĺ�18���ӵķ���ΪH2O2���÷��ӵĵ���ʽΪ��![]() ��DΪNaԪ�أ�������D2WΪNa2O�� �õ���ʽ��ʾ�����Ƶ��γɹ���Ϊ��
��DΪNaԪ�أ�������D2WΪNa2O�� �õ���ʽ��ʾ�����Ƶ��γɹ���Ϊ��![]() ���𰸣�
���𰸣� ��
��![]() ��
��
(3) a.W����ΪO2������H2S��Һ��Ӧ��O2+2H2S= S![]() +H2O����Һ����ǣ�����Ϊ����������ԭ��Ӧ�������ʣ����ʵ�������Oǿ��S���ǽ�����Oǿ��S����a��ȷ��b���ǽ�����ǿ�����䵥�ʵĵõ������ף������ǵõ��ӵ���Ŀ����b����c��WΪOԪ�أ��ǽ�����Խǿ�����⻯��Խ�ȶ�������O��S��Ԫ�صļ��⻯�����ȷֽ⣬ǰ�ߵķֽ��¶ȸߣ���˵��O�ķǽ�����ǿ����c��ȷ���𰸣�a��c��
+H2O����Һ����ǣ�����Ϊ����������ԭ��Ӧ�������ʣ����ʵ�������Oǿ��S���ǽ�����Oǿ��S����a��ȷ��b���ǽ�����ǿ�����䵥�ʵĵõ������ף������ǵõ��ӵ���Ŀ����b����c��WΪOԪ�أ��ǽ�����Խǿ�����⻯��Խ�ȶ�������O��S��Ԫ�صļ��⻯�����ȷֽ⣬ǰ�ߵķֽ��¶ȸߣ���˵��O�ķǽ�����ǿ����c��ȷ���𰸣�a��c��
��4��XΪNԪ�أ�X������������Ӧˮ����ΪHNO3������̬�⻯��ΪNH3��HNO3��NH3���γɵĻ�����ΪNH4NO3��NH4NO3�к��еĻ�ѧ�����������Ӽ����ۼ����𰸣����Ӽ����ۼ���
��5��˫��ˮ���������ԣ���������ͭ���ʸ÷�Ӧ�����ӷ���ʽΪ��Cu+H2O2+2H+=Cu2+ +2H2O���𰸣�Cu+H2O2+2H+=Cu2+ +2H2O��

 ��������ܸ�ϰϵ�д�
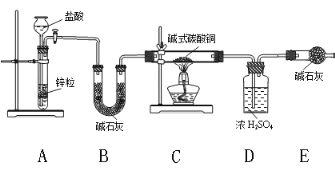
��������ܸ�ϰϵ�д�����Ŀ������ͼװ����ȡ���е����ָ�����������壨ͼ������̨�����С����ȼ������ռ�װ�þ�����ȥ����Ҫʱ���Լ��ȣ�a��b��c��d��ʾ��Ӧ�����м�����Լ�����������ȷ���ǣ� ��

ѡ�� | ���� | a | b | c | d |
A | CO2 | ���� | CaCO3 | ����Na2CO��Һ | Ũ���� |
B | Cl2 | Ũ���� | MnO2 | NaOH��Һ | Ũ���� |
C | NH3 | ����NH4Cl��Һ | ��ʯ�� | H2O | ����NaOH |
D | NO | ϡ���� | ͭм | H2O | Ũ���� |
A. A B. B C. C D. D