��Ŀ����
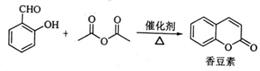
��ѧ�ϳ���ȼ�շ�ȷ���л�����ɣ����ַ������ڵ�¯����ʱ�ô���������������Ʒ�����ݲ��������ȷ���л������ɡ��л���M(����ʽ��CxHySz)�����ηɻ����������ϵ���Ҫ�ɷ֡�
M���ȼ�յIJ���Ϊ���������д����ѧ����ʽ��_______ _______��
����ʵ�飺
ij��ѧ��ȤС��Ϊ��֤M���Ԫ�ؽ���������ʵ��:��������Ʒ����ȼ�չ�A�У�ͨ��һ����O2���õ�¯����ʹ��ȼ��,����װ����ͼ��ʾ(�г�������װ������ȥ)��
��1����ʵ��װ������˳��Ϊ____________________��������ÿһ������ֻ��ʹ��һ�Σ�
��2��D��ʢ�ŵ��Լ���________
��3����֤���л��ﺬ̼Ԫ�ص�������_________________________________________��
��4��ȼ�չ��з���CuO��������________________________________��
��5��ָ�������д����װ�ã�__________________________________________________��
����ʵ�飺
��1���������CO2���������������ͼ��ʾװ�ã�ʵ�������ٵ�����Ͳ����Һ��߶�ʹ֮��ͬ������ȴ�����£��۶�ȡ��Ͳ��������������������������ȷ˳���ǣ�________������д������ţ���
��2���������CO2�������������ó����������г�������õ���
a��0.1mol/LCaCl2��Һ b��0.1mol/L Ca(OH)2��Һ
c��0.1mol/L Ba(NO3)2��Һ d��0.1mol/L Ba(OH)2��Һ
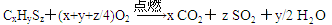
I��1��A��C��B����D����D����B����E
��2�����Ը��������Һ
��3��D����B������Һ����ɫ��E����Һ�����
��4�����л����е�̼Ԫ��ȫ������Ϊ������̼
��5��װ��E���Լ�ƿδ�������ͨ
II��1���ڢ٢ۣ�2��d
��������������������л���M�ķ���ʽΪCxHySz�����ȼ�յIJ���Ϊ���������TCO2��SO2��H2O���ݴ���дȼ�շ���ʽ��
I�����������֪������Ӧ����Ʒ����A��ͨ���������������к���CO2��SO2��H2O�ȣ���Ʒ������Ը��������Һ���鲢����SO2���ó���ʯ��ˮ����CO2������װ������˳��ΪA��C��B��D��E��A��C��D��B��E��D��ʢ�ŵ��Լ������Ը��������Һ��֤���л��ﺬ̼Ԫ�ص�����ΪD����B������Һ����ɫ��E����Һ(����ʯ��ˮ)����ǣ�ȼ�չ��з���CuO�������ǽ��л����е�̼Ԫ��ȫ������Ϊ������̼��װ���еĴ���װ��E���Լ�ƿδ�������ͨ��
II����1���ⶨ����CO2��������������Ӧ��������������ȴ�����£�Ȼ�������Ͳ����Һ��߶�ʹ֮��ͬ�Լ�С������������2�����������Լ��У�bd������CO2���ɳ�����������BaCO3��Ħ������������ⶨ�����Խ�С������ѳ�����Ϊd��
���㣺����ȼ�շ��ⶨ�л������ʽ�����֪ʶ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����������һ����;�㷺�ľ�ϸ������Ʒ��ij����С�����ʵ������ȡ���ᴿ���������ķ������£�
��֪�����Ȼ��ƿ����Ҵ��γ�CaCl2��6C2H5OH
���й��л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/�� | 34.7 | 78.5 | 118 | 77.1 |
��2CH3CH2OH
 CH3CH2OCH2CH3��H2O
CH3CH2OCH2CH3��H2O I���Ʊ�����
װ����ͼ��ʾ��A�з���Ũ���ᣬB�з���9.5mL��ˮ�Ҵ���6mL�����ᣬD�з��б���̼������Һ��
��1��д���������Ҵ�����������Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ������еμӴ�Լ3mLŨ���ᣬB���ݻ�����ʵ��� �������� ��������ȷѡ��ǰ����ĸ��
A��25mL B��50mL C��250mL D��500mL
��3�����θ���ܵ���Ҫ������ ��
��4��Ԥ����Na2CO3��Һ�еμӼ��η�̪��Һ��Ŀ����
��
���ᴿ�������ٽ�D�л��Һת���Һ©�����з�Һ��
���л�����5mL����ʳ��ˮϴ�ӣ�����5mL�����Ȼ�����Һϴ�ӣ������ˮϴ�ӡ��л��㵹��һ�������ƿ�У�����ˮ����þ����ôֲ��
�۽��ֲ��������ռ�77.1�����֣��õ��������������������
��5���ڢٲ���Һʱ��ѡ�õ����ֲ������������Ʒֱ��� �� ��
��6���ڢڲ����ñ���ʳ��ˮ�������Ȼ�����Һ�������ˮϴ�ӣ��ֱ���Ҫϴȥ�ֲ�Ʒ�е� �� �� ��
ʵ������ȼ�շ��ⶨij�ְ�����(CxHyOzNp)�ķ�����ɡ�ȡn g���ְ�������ڴ������г��ȼ�գ�����CO2��H2O��N2���ְ�ͼ��ʾװ�ý���ʵ�飺
��ش������й����⣺
(1)ʵ�鿪ʼʱ������Ҫͨ��һ��ʱ�����������������__________________________��
(2)����װ������Ҫ���ȵ�������________(����ĸ���)������ʱӦ�ȵ�ȼ________���ľƾ��ơ�
(3)Aװ���з�����Ӧ�Ļ�ѧ����ʽ��__________________________________________��
(4)װ��D��������___________________________________��
(5)��ȡN2���ʱ��Ӧע�⣺
��________________________________________________��
��________________________________________________��
(6)ʵ���в��N2�����ΪV mL(������Ϊ��״��)��Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������________(����ĸ���)��
| A������CO2��������� |
| B������H2O������ |
| C��ͨ��O2����� |
| D�����������Է������� |
��ϩ������ʯ�͵���Ҫ�л�����ԭ�ϣ������ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ���������·�ش�
��֪��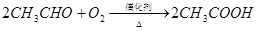
��1����ӦII�Ļ�ѧ����ʽ�� ��
��2��DΪ�߷��ӻ������������������ְ�װ���ϣ���ṹ��ʽ�� ��
��3��E������ζ�����ʣ���ʵ��������ͼװ����ȡ��
�ٷ�ӦIV�Ļ�ѧ����ʽ�� ���÷�Ӧ����Ϊ ��
�ڸ�װ��ͼ����һ�����ԵĴ����� ��
��4��Ϊ��֤��Ũ�����ڷ�ӦIV�����˴�������ˮ�������ã�ijͬѧ������ͼ�Ľ���װ�ý���������4��ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ����ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܼ����Լ� | �Թ������Լ� | �л���ĺ��/cm |
| A | 2 mL�Ҵ���1 mL���ᡢ 1mL18mol��L��1Ũ���� | ����Na2CO3��Һ | 3.0 |
| B | 2 mL�Ҵ���1 mL���� | 0.1 | |
| C | 2 mL�Ҵ���1 mL���ᡢ 3 mL 2mol��L��1 H2SO4 | 0.6 | |
| D | 2 mL�Ҵ���1 mL���ᡢ���� | 0.6 |
��ʵ��D��Ŀ������ʵ��C����գ�֤��H+��������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ���3mL�� mol��L��1��
�ڷ���ʵ�� ����ʵ���ţ������ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʡ�
��֪��CH3CH2OH CH2=CH2��+H20
CH2=CH2��+H20
CH2=CH2+Br2 BrCH2��CH2Br
BrCH2��CH2Br
���Ҵ���1,2-�������顢���ѵ��й������������±���ʾ��
| | �Ҵ� | 1,2-�������� | ���� |
| ͨ��״���µ�״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g��cm-3 | 0.79 | 2.2 | 0.71 |
| �۵�/�� | -130 | 9 | -116 |
| �е�/�� | 78.5 | 132 | 34.6 |
ij��ѧС�������Ҵ������Ϊԭ����ʵ�����Ʊ�������1,2һ�������飬ʵ��װ������ͼ��ʾ(װ��A�еļ��Ȳ��ֵ�����װ��ʡ��û�л���)��

��1������E�������� ��
��2����������©�������IJ����ܵ��� ���� (����ĸ)��
a��ʹ©����Һ��������
b��������������������
c����ֹA��������ƿ���Һ�屬��
��3��ʵ��ʱ��A��������ƿ����뼸Ƭ���Ƭ��Ŀ���� �����ȷ�Ӧ�����У�������ƿ���������ϩ�⣬���������ɵ��л���������Ҫ�� ��
��4����Ӧ�����У���B�г���������(��)���Һ����������˵������������ (�D�г��ֶ�������C�������ѳ�����)��ɵġ�
��5����Ӧ�����У�D��������ˮ��ȴʢ��Һ����Թܣ�����ҪĿ���� ��˵���Ʊ�1,2-��������ķ�Ӧ�Ѿ�������ʵ�������� ��
����һ��˫����ϩ��������ӳɺ�IJ���ṹ��ʽ��ͼ������������еĽṹ�У� ��
| A��4�� | B��5�� | C��6�� | D��7�� |