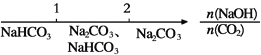��Ŀ����
����Ŀ��I��������̼�ǵ�������ЧӦ��������ף�Ŀǰ���Ǵ���������̼�ķ���֮һ��ʹ����������Ӧ�ϳɼ״�����֪�������״�ȼ�յ��Ȼ�ѧ����ʽ���£�
��2H2(g)+O2(g)=2H2O(l) ��H=- 283.0kJ��mol;
��2CH3OH (1)+3O2=2CO2(g)+4H2O(1) ��H=- 726.0kJ��mol��
(1)д��������̼�������ϳɼ״�Һ����Ȼ�ѧ����ʽ____��
(2)��֪��CO2 (g)+3H2 (g)=CH3OH (g)+H2O(g) ��H=- 49.0kJ��mol����6molCO2��8mol H2����4L���ܱ������У����H2�����ʵ�����ʱ��仯��ͼ��ʾ��ʵ�ߣ���ͼ������a(l��6)��ʾ��1minʱH2�����ʵ�����6mol��

��b������Ӧ����____������������������������С�������淴Ӧ���ʡ����¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ____��
�ڽ��ı�ijһʵ�������ٽ�������ʵ����H2�����ʵ�����ʱ��仯��ͼ��������ʾ����������Ӧ��ʵ�������ı���____����������ٳ���3 mol CO2��4mol H2��H2O(g)���������____��������������������������С����
��.������Ʒ�к�Fe��Zn��Ag��Cu�����ֽ������ʣ�Ϊ��øߴ��ȵ�����ij��ȤС��ͬѧ����Ǧ����Ϊ��Դ��������ʯīΪ�缫�������������Һ�Դ��������ᴿ������֪�������ԣ�Fe2+<Ni2+<Cu2+��
(1)Ǧ���صĸ����缫��ӦʽΪ ___��
(2)�����ᴿ��������ӦʽΪ ___�����������������������ij��������Ҫ�ɷ�Ϊ____��
���𰸡� CO2��g��+3H2��g��=CH3OH��l��+H2O��l����H=-61.5kJmol-1 ���� 2L2/mol2 �����¶� ���� Pb+SO42--2e-=PbSO4 Ni2++2e-=Ni Ag��Cu
��������I��(1)��2H2(g)+O2(g)=2H2O(l)����H=-283.0kJmol-1����2CH3OH(l)+3O2(g)��2CO2(g)+4H2O(l) ��H=-726.0kJmol-1�����ݸ�˹���ɢ���3-�ڵõ���2CO2 (g)+6H2 (g)=2CH3OH(l)+2H2O (l) ��H=-123kJmol-1���Ȼ�ѧ����ʽΪ��CO2 (g)+3H2 (g)=CH3OH(l)+H2O (l) ��H=-61.5kJmol-1���ʴ�Ϊ��CO2 (g)+3H2 (g)=CH3OH(l)+H2O (l) ��H=-61.5kJmol-1��
(2)�ٸ���ͼ���֪����b��ʱ����Ӧû�дﵽ��ѧƽ�⣬��ʱ��Ӧ��������Ӧ��������ţ�����v����v���������ķ�ӦΪ��CO2(g)+3H2(g)CH3OH(g)+H2O(g)���䷴Ӧ��ƽ�ⳣ��ΪK��![]() ��ƽ��ʱ������ͼ��ͷ�Ӧ�ķ���ʽ��֪��c(H2)��
��ƽ��ʱ������ͼ��ͷ�Ӧ�ķ���ʽ��֪��c(H2)��![]() ��0.5mol/L��c(CO2)��
��0.5mol/L��c(CO2)��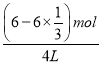 ��1mol/L��c(CH3OH)��
��1mol/L��c(CH3OH)��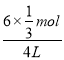 ��0.5mol/L��c(H2O)��
��0.5mol/L��c(H2O)��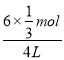 ��0.5mol/L���ֱ����ƽ�ⳣ�������K��
��0.5mol/L���ֱ����ƽ�ⳣ�������K��![]() ��2L2/mol2���ʴ�Ϊ�����ڣ�2L2/mol2��
��2L2/mol2���ʴ�Ϊ�����ڣ�2L2/mol2��
����֪��ӦCO2(g)+3H2(g)CH3OH(g)+H2O(g)�����ʱ���H��0���۲�����II���ﵽƽ���ʱ��䳤����ƽ��ʱ�������٣�ƽ�������ƶ���������ߢ��Ӧ��ʵ�������ı��ǽ����¶ȣ���������ٳ���3molCO2��4molH2�������˷�Ӧ��Ũ�ȣ���ѧƽ��������Ӧ�����ƶ������ɸ����H2O(g)��������������䣬��ȻH2O(g)������������ʴ�Ϊ�������¶ȣ�����
II��(1)Ǧ���صĸ���ΪPb���缫��ӦʽΪPb+SO42--2e-=PbSO4���ʴ�Ϊ��Pb+SO42--2e-=PbSO4��
(2)�����ᴿʱ��������Ni2+�õ���������Ni���缫��ӦʽΪNi2++2e-=Ni�������Ϸ����ķ�ӦΪ����ʧȥ���ӱ���������ԭ��Խǿ�Ľ���������ʧȥ���ӱ�������������Ʒ�к�Fe��Zn��Ag��Cu�����ֽ������ʣ��������ӵ�������Խǿ��������Ļ�ԭ��Խ�������������ԣ�Fe2+ <Ni2+ <Cu2+֪�����������������������ij��������Ҫ�ɷ�ΪAg��Cu���ʴ�Ϊ��Ni2++2e-=Ni��Ag��Cu��

����Ŀ����Ԫ�ظ�(Cr)����Һ����Ҫ��Cr3+(����ɫ)��Cr(OH)4(��ɫ)��Cr2O72(�Ⱥ�ɫ)��CrO42(��ɫ)����ʽ���ڣ�Cr(OH)3Ϊ������ˮ�Ļ���ɫ���壬�ش��������⣺
��1��Cr3+��Al3+�Ļ�ѧ�������ơ���Cr2(SO4)3��Һ����μ���NaOH��Һֱ���������ɹ۲쵽��������_________��
��2��+6�۸��Ļ����ﶾ�Խϴ���NaHSO3�����Է�Һ�е�Cr2O72��ԭ��Cr3+���÷�Ӧ�����ӷ���ʽΪ______________��
���Թ�����Ϊ�����������������V2O5���ǽӴ�����������Ĵ������ӷϷ������л���V2O5�ȱ�����Ⱦ��������������Դ�ۺ����á��Ϸ���������Ҫ�ɷ�Ϊ��
���� | V2O5 | V2O4 | K2SO4 | SiO2 | Fe2O3 | Al2O3 |
��������/% | 2.2~2.9 | 2.8~3.1 | 22~28 | 60~65 | 1~2 | <1 |
������һ�ַϷ��������չ���·�ߣ�
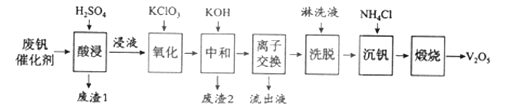
��1���������ʱV2O5ת��ΪVO2+����Ӧ�����ӷ���ʽΪ___________
��2�����������õ�ƫ����泥�NH4VO3��������д�������ա��з�����Ӧ�Ļ�ѧ����ʽ____________��
III������пΪ��ɫ��ĩ��������ʪ�Ѣ��Ƥ���������ơ�������ҵ������п(����Fe(��), Mn(��), Ni(��)������)����������:

�ڱ�ʵ�������£�Ni(��)���ܱ�������������صĻ�ԭ������MnO2���ش���������:
��Ӧ���з�����Ӧ�����ӷ���ʽΪ___________��___________��