��Ŀ����
����Ŀ��ͭ���ʼ��仯�����ںܶ���������Ҫ����;��
I.�����ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��![]() ������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�
������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�
(1)��ϸͭ�۵�ij�Ʊ�������ͼ��ʾ��![]() �������Ļ�ѧ���� ______ ��
�������Ļ�ѧ���� ______ ��
![]()
(2)�Ȼ���ͭ![]() ���Ʊ������ǣ���
���Ʊ������ǣ���![]() ��Һ��ͨ��һ����
��Һ��ͨ��һ����![]() ���ȣ���Ӧһ��ʱ�������CuCl��ɫ��������Ӧ�����ӷ���ʽΪ ______ ��
���ȣ���Ӧһ��ʱ�������CuCl��ɫ��������Ӧ�����ӷ���ʽΪ ______ ��
II.������Һ��һ�ֱ�����ɱ�������㷺Ӧ������ľ�������ͻ����ϣ�����ɫ�ĵ������������Ʋ�����Һ����Ҫԭ�ϣ���֪![]() �IJ��ֽṹ�ɱ�ʾ���£�
�IJ��ֽṹ�ɱ�ʾ���£�

(1)д��ͭԭ�Ӽ۵��Ӳ�ĵ����Ų�ʽ ______ ����ͭͬ���ڵ�����Ԫ�صĻ�̬ԭ����������������ͭԭ����ͬ��Ԫ���� ______ ![]() ��Ԫ�ط���
��Ԫ�ط���![]() ��
��
(2)������ͼ�а�![]() �ṹ�еĻ�ѧ����ʾ������_____________
�ṹ�еĻ�ѧ����ʾ������_____________
(3)![]() ��������λ���� ______ ���ӻ����������
��������λ���� ______ ���ӻ����������![]() �ӻ���ԭ���� ______ ��
�ӻ���ԭ���� ______ ��
(4)��������Cu��ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��
��֪![]() ���������ӵ�������Cu�����ԭ������ΪM��Cu���ʾ�����ܶ�Ϊd
���������ӵ�������Cu�����ԭ������ΪM��Cu���ʾ�����ܶ�Ϊd![]() �þ���Ŀռ��������� ______ ��Cuԭ�Ӱ뾶�ı���ʽΪ ______
�þ���Ŀռ��������� ______ ��Cuԭ�Ӱ뾶�ı���ʽΪ ______ ![]() �ú�
�ú�![]() ��M��d�Ĵ���ʽ��ʾ
��M��d�Ĵ���ʽ��ʾ![]()
���𰸡����Ӽ������ۼ�����λ�� ![]()
![]() K��Cr
K��Cr ![]()
![]() N��S��O
N��S��O ![]()
![]()
��������
![]() �������Ӵ������Ӽ����ǽ���Ԫ�ؼ����γɹ��ۼ���������д�����λ����
�������Ӵ������Ӽ����ǽ���Ԫ�ؼ����γɹ��ۼ���������д�����λ����
![]() ���ݷ�Ӧ�������ͷ�Ӧ����д����Ӧ�ķ�Ӧ����ʽ��
���ݷ�Ӧ�������ͷ�Ӧ����д����Ӧ�ķ�Ӧ����ʽ��
![]() ͭ��29��Ԫ�أ����ݺ�������Ų�������дͭԭ�Ӽ۲���ӵĹ���Ų�ʽ�����ж���ͭͬ���ڵ�����Ԫ�صĻ�̬ԭ����������������ͭԭ����ͬ��Ԫ�أ�
ͭ��29��Ԫ�أ����ݺ�������Ų�������дͭԭ�Ӽ۲���ӵĹ���Ų�ʽ�����ж���ͭͬ���ڵ�����Ԫ�صĻ�̬ԭ����������������ͭԭ����ͬ��Ԫ�أ�
![]() ͭ���ӵ���λ��Ϊ4����4��ˮ�����γ���λ����һ��Oԭ����2��Hԭ���γ�ˮ���������4��Oԭ����Sԭ���γɹ��ۼ���
ͭ���ӵ���λ��Ϊ4����4��ˮ�����γ���λ����һ��Oԭ����2��Hԭ���γ�ˮ���������4��Oԭ����Sԭ���γɹ��ۼ���
![]() ��Nԭ�Ӻ��йµ��Ӷ������壬���ݷ���������ԭ�ӵļ۲���Ӷ����жϣ�
��Nԭ�Ӻ��йµ��Ӷ������壬���ݷ���������ԭ�ӵļ۲���Ӷ����жϣ�
![]() �ľ���Ϊ��������������
�ľ���Ϊ��������������![]() ����ԭ�Ӱ뾶��
����ԭ�Ӱ뾶��
![]() �������Ӵ������Ӽ����ǽ���Ԫ�ؼ����γɹ��ۼ���������д�����λ��������
�������Ӵ������Ӽ����ǽ���Ԫ�ؼ����γɹ��ۼ���������д�����λ��������![]() �������Ļ�ѧ�������Ӽ������ۼ�����λ����
�������Ļ�ѧ�������Ӽ������ۼ�����λ����
�ʴ�Ϊ�����Ӽ������ۼ�����λ����
![]() �÷�Ӧ�ж�����������ԭ��������������ӣ�ͭ���ӵõ���������ͭ���ӣ���Ӧ�����Ǽ��ȣ����Ը÷�Ӧ�����ӷ���ʽΪ��
�÷�Ӧ�ж�����������ԭ��������������ӣ�ͭ���ӵõ���������ͭ���ӣ���Ӧ�����Ǽ��ȣ����Ը÷�Ӧ�����ӷ���ʽΪ��![]() ��
��
![]() ͭ��29��Ԫ�أ���������Ų�ʽΪ
ͭ��29��Ԫ�أ���������Ų�ʽΪ![]() ����̬ͭԭ�Ӽ۵��Ӳ�ĵ����Ų�ʽΪ
����̬ͭԭ�Ӽ۵��Ӳ�ĵ����Ų�ʽΪ![]() ��ͭԭ��������Ų�Ϊ
��ͭԭ��������Ų�Ϊ![]() ��ͬ��������Ų�Ϊ
��ͬ��������Ų�Ϊ![]() �� 3d�ܼ�û�е��ӵ�Ԫ��ΪK��3d�ܼ�����5������ΪCr��
�� 3d�ܼ�û�е��ӵ�Ԫ��ΪK��3d�ܼ�����5������ΪCr��
�ʴ�Ϊ��![]()
![]() ͭ���ӵ���λ��Ϊ4����4��ˮ�����γ���λ����һ��Oԭ����2��Hԭ���γ�ˮ���������4��Oԭ����Sԭ���γɹ��ۼ�����
ͭ���ӵ���λ��Ϊ4����4��ˮ�����γ���λ����һ��Oԭ����2��Hԭ���γ�ˮ���������4��Oԭ����Sԭ���γɹ��ۼ�����![]() �ṹ�еĻ�ѧ����ʾΪ��
�ṹ�еĻ�ѧ����ʾΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
![]() ��Nԭ�Ӻ��йµ��Ӷ������壬
��Nԭ�Ӻ��йµ��Ӷ������壬![]() ��������λ����
�����������![]() ��
��![]() ��S��O�γ�4��
��S��O�γ�4��![]() ����û�й¶Ե��ӣ���
����û�й¶Ե��ӣ���![]() �ӻ���
�ӻ���![]() ��N��H�γ�3��
��N��H�γ�3��![]() ������1�Թ¶Ե��ӣ���۲���Ӷ���Ϊ4����
������1�Թ¶Ե��ӣ���۲���Ӷ���Ϊ4����![]() �ӻ���
�ӻ���![]() ��O��H�γ�2��
��O��H�γ�2��![]() ������2�Թ¶Ե��ӣ���۲���Ӷ���Ϊ4����
������2�Թ¶Ե��ӣ���۲���Ӷ���Ϊ4����![]() �ӻ���������ԭ����
�ӻ���������ԭ����![]() �ӻ���ԭ����N��O��S��
�ӻ���ԭ����N��O��S��
�ʴ�Ϊ��![]() ��N��S��O��
��N��S��O��
![]() ����ͭ���������������ܶѻ�������Ŀռ���������
����ͭ���������������ܶѻ�������Ŀռ���������![]() ��������Cuԭ����ĿΪ
��������Cuԭ����ĿΪ![]() ����ͭԭ�ӵİ뾶Ϊrcm�������ⳤΪ
����ͭԭ�ӵİ뾶Ϊrcm�������ⳤΪ![]() ��
��
����![]() �����
�����![]() ��
��
�ʴ�Ϊ��![]() ��
��![]() ��
��

����Ŀ����������������Ӧ�ù㷺��
(1) NH2Cl��ˮ��Ӧ����ǿ�����Ե����ʣ�������Ч��������������ҵ�Ͽ�ͨ����Ӧ��NH3(g)+Cl2(g)=NH2Cl(g) + HCl(g)�Ʊ��Ȱ�����֪���ֻ�ѧ���ļ������±���ʾ(�ٶ���ͬ������ͬ�ֻ�ѧ���ļ���һ��)�� ��������Ӧ��H=__________kJ��mol��1
��ѧ�� | N-H | Cl-Cl | N-Cl | H-Cl |
����/(kJ/mol) | a | b | c | d |
(2)��������Ҫ�Ļ�����Ʒ��Ŀǰ��ҵ�ϳɰ���ԭ����: N2(g)+3H2(g) ![]() 2NH3(g)���ں��º�ѹװ���н��й�ҵ�ϳɰ���Ӧ������˵����ȷ����__________��
2NH3(g)���ں��º�ѹװ���н��й�ҵ�ϳɰ���Ӧ������˵����ȷ����__________��
a.����ѹǿ���ٱ仯ʱ�������÷�Ӧ�Ѵ�ƽ��״̬
b.�����ܶȲ��ٱ仯ʱ�������÷�Ӧ�Ѵ�ƽ��״̬
c.ƽ���ѹ�������������ɸ���NH3
d.ƽ�����װ����ͨ��һ����Ar�� ƽ�ⲻ�ƶ�
(3)�������������Ϊ5L,�¶ȷֱ�㶨ΪT1�� T2��T3�ĺ����ܱ�����I��II�� III�У��ֱ����1 mol N2��3 molH2������Ӧ: N2(g)+3H2(g) ![]() 2NH3(g) H1=-93 kJ��mol��1������Ӧ�����е�2minʱH2�����������ͼ��ʾ������ֻ��һ�������еķ�Ӧ�Ѿ��ﵽƽ��״̬��
2NH3(g) H1=-93 kJ��mol��1������Ӧ�����е�2minʱH2�����������ͼ��ʾ������ֻ��һ�������еķ�Ӧ�Ѿ��ﵽƽ��״̬��
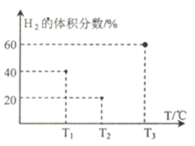
��2minʱ���������еķ�Ӧ�ﵽ��ѧƽ��״̬����_____����.(����I������II������III��)��
��0��2 min������I����NH3��ʾ�Ļ�ѧ��Ӧ����v(NH3)=____�� (������λ��Ч����)
��2 minʱ����II��v��______ v���� (����<���� ��>������=��)
�������������еķ�Ӧ���ﵽƽ��״̬ʱ��ƽ�ⳣ����С��������______(���������)��������ֵΪ____(������λ��Ч����)��
(4)���ڸ����¿ɽ�һЩ������������ﻹԭΪ��̬��Һ̬�������ʣ�����������ΪN2���ڲ�ͬ�¶��£�������ԭ���ֽ���������ﵽƽ���������![]() ���¶�(T)�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����______(����ĸ)��
���¶�(T)�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����______(����ĸ)��
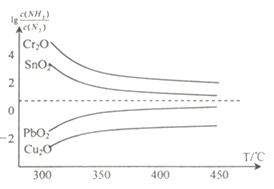
A. NH3��ԭPbO2�ķ�Ӧ��H>0
B.��ҵұ�������ֽ���ʱ��NH3ұ��������(Cr)�Ļ�ԭЧ�����
C.ʵ���һ�ԭ������ͭ(Cu)ʱ��325��C ��NH3�������ʱ�425��C��NH3�������ʸ���
D.ͨ���ӳ���Ӧ�ܵij��������ӽ����������NH3�ĽӴ���������Լ���β����NH3����

