��Ŀ����
����Ŀ�������������������ؾ��壨K3[Fe(C2O4)3]3H2O��������Ϊ�л���Ӧ�Ĵ���������ˮ���������Ҵ���ʵ���ҿ���Ħ���ε�Ϊԭ���Ʊ�������������ͼ��
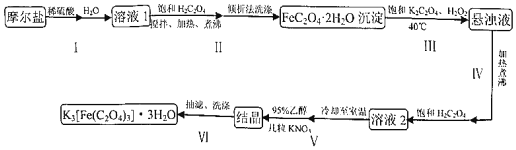
��1����ʵ�黯ѧ�����Ʊ�Ħ���εĻ�ѧ����ʽΪ______
��2������1������ϡ�����������______���������й������Ͳ����������______
��3�������ʵ�鷽��֤��FeC2O42H2O�����Ѿ�ϴ�Ӹɾ���______
��4����������40�������½��е�ԭ��______
��5��������������ͼ��װ��װ�ã��밴��ȷ����˳��������
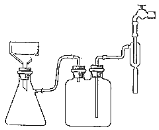
�ڲ���©���м�����ֽ��______��ȷ�ϳ��
a��ת�ƹ�Һ����b������ˮ��ͷ��c���ر�ˮ��ͷ��d����ˮ��ͷ��e��������������ˮ��ʪ��ֽ
���𰸡�(NH4)2SO4+FeSO4+6H2O=(NH4)2Fe(SO4)26H2O�� ��ֹFe2+ˮ�� ʹFe2+��ȫת��ΪFeC2O42H2O���� ȡ���һ��ϴ��Һ������BaCl2��Һ�����ް�ɫ������˵���Ѿ�ϴ�Ӹɾ� �¶�̫��Fe2+ˮ����������̫�����¶ȹ�������H2O2�ֽ� e��d��a��b
��������
��1����ʵ�黯ѧ�����Ʊ�Ħ���εĻ�ѧ����ʽΪ����NH4��2SO4+FeSO4+6H2O=(NH4)2Fe(SO4)26H2O��;�ʴ�Ϊ��(NH4)2SO4+FeSO4+6H2O=(NH4)2Fe(SO4)26H2O����
��2��Fe2+��ˮ�⣬��Ϊ��ֹFe2+ˮ�⣬Ħ���ε��ܽ��м���������ϡ���������й������Ͳ���ʹFe2+��ȫת��ΪFeC2O42H2O�������ʴ�Ϊ����ֹFe2+ˮ�⣻ʹFe2+��ȫת��ΪFeC2O42H2O������
��3��FeC2O42H2O���帽����������ӣ�ȡ���һ��ϴ��Һ������BaCl2��Һ�����ް�ɫ������˵���Ѿ�ϴ�Ӹɾ����ʴ�Ϊ��ȡ���һ��ϴ��Һ������BaCl2��Һ�����ް�ɫ������˵���Ѿ�ϴ�Ӹɾ���
��4���¶�̫��Fe2+ˮ����������̫�����¶ȹ�������H2O2�ֽ⣬�ʲ������40�������½��У� �ʴ�Ϊ���¶�̫��Fe2+ˮ����������̫�����¶ȹ�������H2O2�ֽ⣻
��5�����˵IJ���Ϊ���ڲ���©���м�����ֽ��������������ˮ��ʪ��ֽ����ˮ��ͷ��ת�ƹ�Һ�����������ˮ��ͷ��ȷ�ϳ�ɣ��ε���Ƥ�ܣ��ر�ˮ��ͷ���ʴ�Ϊ��e��d��a��b��

 53���ò�ϵ�д�
53���ò�ϵ�д�