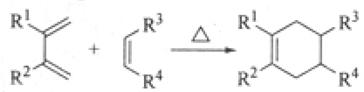��Ŀ����
��ÿ��2�֣���14�֣�
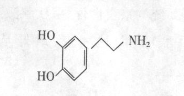
�й��ǡ�������Э���顷�ĵ�Լ����һ�ᷴ��ʹ�û�ѧ�����������κ���ʽ�Ļ�ѧ������ɢ��������ͪ��һ�־��кɻ���ζ�Ҿ���ǿ�������õĻ�ѧ��
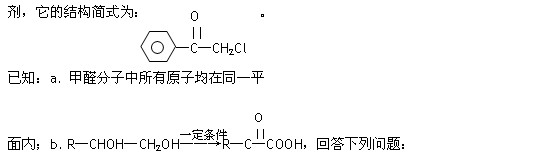
(1)������ͪ�����У���ͬһƽ���ڵ�̼ԭ������� ����
(2)������ͪ�����ܷ����Ļ�ѧ��Ӧ�� ��
(3)�������б����������������ܷ���������Ӧ�ı�����ͪ��ͬ���칹���� �֡�
(4)������ͪ��һ��ͬ���칹��������ת����ϵ��
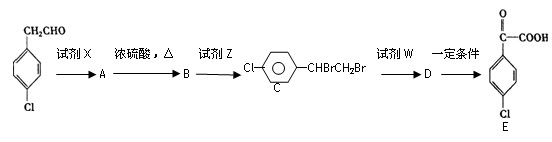
�����
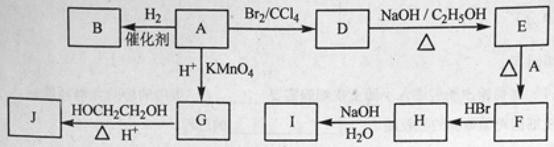
��B�Ľṹ��ʽ�� ���Լ�XΪ ��
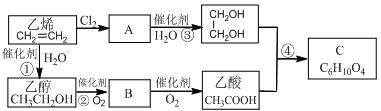
������ת���Ļ�ѧ����ʽA��B ��
A��E��F ��
�й��ǡ�������Э���顷�ĵ�Լ����һ�ᷴ��ʹ�û�ѧ�����������κ���ʽ�Ļ�ѧ������ɢ��������ͪ��һ�־��кɻ���ζ�Ҿ���ǿ�������õĻ�ѧ��

(1)������ͪ�����У���ͬһƽ���ڵ�̼ԭ������� ����
(2)������ͪ�����ܷ����Ļ�ѧ��Ӧ�� ��
| A���ӳɷ�Ӧ | B��ȡ����Ӧ | C����ȥ��Ӧ | D��ˮ�ⷴӦ E��������Ӧ |
(4)������ͪ��һ��ͬ���칹��������ת����ϵ��

�����
��B�Ľṹ��ʽ�� ���Լ�XΪ ��
������ת���Ļ�ѧ����ʽA��B ��
A��E��F ��
��

��ϰ��ϵ�д�
 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
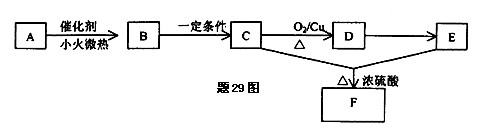
�����Ŀ




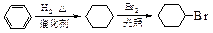 )
)

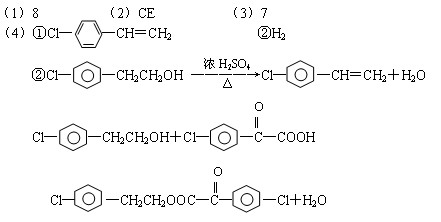
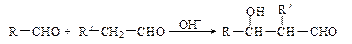 ��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��
��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��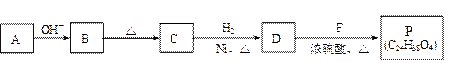
 ����ˮ��ΪM��N b��һ��������M����ת��ΪN
����ˮ��ΪM��N b��һ��������M����ת��ΪN ���Ƴ���������Ϊ���ڸ�����֮�䴫����Ϣ�Ļ�ѧ�����Ƕ�Ͱ������ԡ���������
���Ƴ���������Ϊ���ڸ�����֮�䴫����Ϣ�Ļ�ѧ�����Ƕ�Ͱ������ԡ��������� ���ֳ�Ϊ��Ͱ�ϵͳ����Ͱ��ṹΪ��
���ֳ�Ϊ��Ͱ�ϵͳ����Ͱ��ṹΪ��