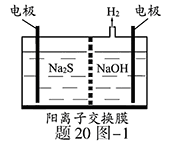
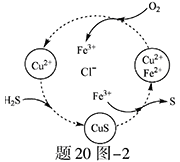
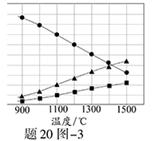
��Ŀ����
ij��Һ���ܺ���Cl-��SO42-��CO32-��NH4+��Fe3+��Al3+��K+��ȡ����Һ100mL���������NaOH��Һ�����ȣ��õ�0��02mol���壬ͬʱ�������ɫ���������ˣ�ϴ�ӣ����գ��õ�1��6g���壻��������Һ�м�����BaCl2��Һ���õ�4��66g����������ij������ɴ˿�֪ԭ��Һ��( )
| A�����ٴ���5������ | B��Cl-һ�����ڣ���c��Cl?����0��4mol/L |
| C��SO42-��NH4+��һ�����ڣ�Cl-���ܲ����� | D��CO32-��Al3+һ�������ڣ�K+���ܴ��� |
B
������������ݼ������NaOH��Һ�����ȣ��õ�0��02mol���壬˵����NH4+������Ϊ0��02mol��ͬʱ�������ɫ������˵����Fe3+��n(Fe3+)=2n(Fe2O3)=2��(1��6g��160g/mol)=0��02mol������ΪFe3+����CO32-����˫ˮ�⣺2Fe3++3CO32-+3H2O=2Fe(OH)3��+3CO2�����CO32-���ܴ������档�ʿ�ȷ��û��CO32-�����ݲ����������4��66g������˵����SO42-��n(SO42-)=n(BaSO4) =4��66g��233g/mol=0��02mol���ٸ��ݵ���غ��֪��Һ��һ����Cl-�������ʵ�������Ϊ��n(Cl-)= 0��02mol��3 +0��02mol��1-0��02mol��2=0��04mol��C(Cl-)��n/V=0��04mol��0��1L=0��4mol/L�����ѡ��B��ȷ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
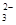

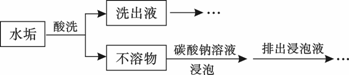
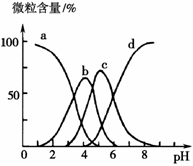
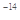 mol
mol ���������������Һ��һ�����Դ���������������ǣ�
���������������Һ��һ�����Դ���������������ǣ�
 S ��n��1��S+ S2��
S ��n��1��S+ S2��