ЬтФПФкШн
ЁОЬтФПЁПАБЁЂСзЪєгкЭЌвЛжїзхдЊЫиЃЌЪЧзщГЩЩњУќЬхЕФживЊдЊЫиЃЌЦфЕЅжЪМАЛЏКЯЮягУЭОЙуЗКЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЛљЬЌPдзгЕФКЫЭтЕчзгХХВМЪНЮЊ_____ЃЌСзМАЦфЭЌжмЦкЯрСкдЊЫиЕквЛЕчРыФмгЩДѓЕНаЁЕФЫГађЪЧ______________________ЁЃ
ЃЈ2ЃЉNH3БШPH3взвКЛЏЕФдвђЪЧ______________________ЁЃ
ЃЈ3ЃЉздШЛЙЬАБЯжЯѓЗЂЩњЕФвЛЯЕСаЛЏбЇБфЛЏЃКN2ЁњNOЁњNO2ЁњHNO3ЁњNO3-НтЪЭСЫУёбшЁАРзгъЗЂзЏМкЁБЕФдРэЁЃ
ЂйN2ЗжзгНсЙЙжаЁЃІвМќКЭІаМќжЎБШЮЊ_________ЃЌМКжЊN![]() NЕФМќФмЮЊ946kJЁЄmol-1ЃЌдЖДѓгкNЁЊNЕФМќФм(193 kJЁЄmol-1)ЕФШ§БЖЃЌдвђЪЧ______________________ЁЃ
NЕФМќФмЮЊ946kJЁЄmol-1ЃЌдЖДѓгкNЁЊNЕФМќФм(193 kJЁЄmol-1)ЕФШ§БЖЃЌдвђЪЧ______________________ЁЃ
ЂкNO3-жаNдзгВЩШЁ___________дгЛЏЗНЪНЃЌЦфПеМфЙЙаЭЮЊ__________ЃЌаДГіЫќЕФвЛжжЕШЕчзгЬхЕФЛЏбЇЪН_____________________ЁЃ
ЂлвбжЊЫсадЃКHNO3>HNO2ЃЌЯТСаЯрЙиМћНтКЯРэЕФЪЧ_________________ЁЃ
A.КЌбѕЫсжабѕдзгзмЪ§дНЖрЃЌЫсаддНЧП
B.ЭЌжждЊЫиЛЏКЯМлдНИпЃЌЖдгІКЌбѕЫсЕФЫсаддНЧП
C.HNO3жаЕЊдЊЫиЕФе§ЕчадИќИпЃЌдкЫЎШмвКжаИќвзЕчРыГіH+ЃЌЫсадЧПгкHNO2
ЃЈ4ЃЉСзЛЏгВ(BP)ЪЧвЛжжГЌгВФЭФЅЭПВуВФСЯЃЌЦфОЇАћНсЙЙШчЭМЫљЪОЃЌЭМжаaЕуКЭbЕуЕФдзгзјБъВЮЪ§вРДЮЮЊЃЈ0ЃЌ0ЃЌ0ЃЉЁЂЃЈ![]() ЃЌ
ЃЌ ![]() ЃЌ
ЃЌ ![]() ЃЉЃЌдђc ЕуЕФдзгзјБъВЮЪ§ЮЊ_______ЁЃвбжЊИУОЇЬхУмЖШЮЊІбgЁЄcm-3ЃЌдђB-PМќЕФМќГЄЮЊ_____pm ЃЈАЂЗќМгЕТТоГЃЪ§гУNAБэЪОЃЌСаГіМЦЫуЪНМДПЩЃЉЁЃ
ЃЉЃЌдђc ЕуЕФдзгзјБъВЮЪ§ЮЊ_______ЁЃвбжЊИУОЇЬхУмЖШЮЊІбgЁЄcm-3ЃЌдђB-PМќЕФМќГЄЮЊ_____pm ЃЈАЂЗќМгЕТТоГЃЪ§гУNAБэЪОЃЌСаГіМЦЫуЪНМДПЩЃЉЁЃ

ЁОД№АИЁП 1s22s22p63s2p3 P>S>Si NH3ЗжзгМфДцдкЧтМќ 1:2 N![]() NжаЕФЕЊдзгДяЕН8ЕчзгЕФЯрЖдЮШЖЈНсЙЙ sp2 ЦНУцШ§НЧаЮ SO3ЁЂO4 BC ЃЈ1ЃЌ
NжаЕФЕЊдзгДяЕН8ЕчзгЕФЯрЖдЮШЖЈНсЙЙ sp2 ЦНУцШ§НЧаЮ SO3ЁЂO4 BC ЃЈ1ЃЌ ![]() ЃЌ
ЃЌ ![]() ЃЉ
ЃЉ  ЁС1010
ЁС1010
ЁОНтЮіЁП(1).PЮЊ15КХдЊЫиЃЌЦфКЫЭтЕчзгЮЊ3Ву5ИіФмМЖЃЌМДЮЊ1s22s22p63s23p3ЃЛЭЌжмЦкгЩзѓЯђгвЕквЛЕчРыФмж№НЅдіДѓЃЌЕЋPЕФ3pФмМЖЮЊАыГфТњзДЬЌЃЌБШНЯЮШЖЈЃЌЫљвдP>S>Si ЃЛ
ЃЈ2ЃЉNH3ЗжзгМфДцдкЧтМќЃЌЗжзгМфзїгУСІНЯЧПЃЌЮЊвзвКЛЏЦјЬхЃЛ
ЃЈ3ЃЉЂйN2ЗжзгаЮГЩЕФЪЧЙВМлШ§МќЃЌЦфжагЩвЛИіІвМќКЭСНИіІаМќЃЌЫљвдІвМќКЭІаМќжЎБШЮЊ1:2ЃЛ
ЛЏбЇМќдНЮШЖЈЃЌМќФмдНДѓЃЌЖјN![]() NжаЕФЕЊдзгДяЕН8ЕчзгЕФЯрЖдЮШЖЈНсЙЙЃЌЫљвдЦфМќФмдЖДѓгкШ§БЖNЁЊNЕФМќФмЁЃ
NжаЕФЕЊдзгДяЕН8ЕчзгЕФЯрЖдЮШЖЈНсЙЙЃЌЫљвдЦфМќФмдЖДѓгкШ§БЖNЁЊNЕФМќФмЁЃ
ЂкЯѕЫсИљжаNдзгМлЕчзгВуЕчзгЮЊ3Жд,РэЯыФЃаЭЮЊЦНУцШ§НЧаЮ,УЛгаЙТЕчзгЖдЃЌЦфдгЛЏЗНЪНЮЊsp2дгЛЏЃЛгыЯѕЫсИљЮЊЕШЕузгЬтЕФЮяжЪЮЊЃКSO3ЁЂO4ЁЂBF3ЕШ
ЂлA. ЭЌжждЊЫиЛЏКЯМлдНИпЃЌКЌбѕЫсжабѕдзгзмЪ§дНЖрЫсаддНЧПЃЌAвдЦЋИХШЋЃЌДэЮѓЃЛB.гЩAЕУЃЌBе§ШЗЃЛC. HNO3жаЕЊдЊЫиЕУЕНЕчзгФмСІЧПЃЌЫљвдЯрНЯHNO2ЫсадИќЧПЁЃCе§ШЗЁЃЫљвдбЁдёBCЁЃ
ЃЈ4ЃЉгЩbЕФзјБъЕУОЇАћБпГЄЮЊ1ЃЌcЮЛгкВрУце§жааФЃЌЫљвдЦфзјБъЮЊЃЈ1ЃЌ ![]() ЃЌ
ЃЌ ![]() ЃЉЃЛгЩвбжЊЕУbДІгк4ИіPдзгЮЇГЩЕФе§ЗНЬхЕФе§жааФЃЌPдзгЮЛгкЖЅЕуЃЌдђB-PМќЕФГЄЖШЮЊ
ЃЉЃЛгЩвбжЊЕУbДІгк4ИіPдзгЮЇГЩЕФе§ЗНЬхЕФе§жааФЃЌPдзгЮЛгкЖЅЕуЃЌдђB-PМќЕФГЄЖШЮЊ![]() ЃЌ
ЃЌ
lЮЊОЇАћБпГЄ= ЃЌЫљвдB-PМќЕФГЄЖШЮЊ
ЃЌЫљвдB-PМќЕФГЄЖШЮЊ pmЁЃ
pmЁЃ

ЁОЬтФПЁПдкИјЖЈЬѕМўЯТЃЌЯТСаРызгзщЕФРызгЙВДцХаЖЯМАЗДгІЕФРызгЗНГЬЪНОље§ШЗЕФЪЧ
бЁЯю | ЬѕМў | Рызгзщ | РызгЙВДцХаЖЯМАЕФРызгЗНГЬЪН |
A |
| Fe2+ЁЂNO3-ЁЂAl3+ЁЂCl- | ВЛФмДѓСПЙВДцЃЌ 3Fe2++4H++NO3-=3Fe3++NO+2H2O |
B | гЩЫЎЕчРыГіc(H+) =1ЁС10-13molЁЄL-1 | K+ЁЂNH4+ЁЂCl-ЁЂAlO2- | ФмДѓСПЙВДц |
C | КЌгаДѓСПAl3+ЕФШмвК | Na+ЁЂNH4+ЁЂSO42-ЁЂCl- | ФмДѓСПЙВДц |
D | ЭЈШыЩйСПSO2ЦјЬх | K+ЁЂNa+ЁЂClO-ЁЂSO42- | ВЛФмДѓСПЙВДцЃЌ 2ClO-+SO2+H2OЃН2HClO+SO32- |
A. A B. B C. C D. D
ЁОЬтФПЁПЙЄвЕЮВЦјжаЕЊбѕЛЏЮяЭЈГЃВЩгУАБДпЛЏЮќЪеЗЈЃЌЦфдРэЪЧNH 3 гыNOxдкДпЛЏМСзїгУЯТЗДгІЩњГЩЮоЖОЕФЮяжЪЁЃФГаЃЛюЖЏаЁзщЭЌбЇВЩгУвдЯТзАжУКЭВНжшФЃФтЙЄвЕЩЯЕЊбѕЛЏЮя ЕФДІРэЙ§ГЬЁЃ

IЃЎЬНОПжЦШЁNH 3 ЕФЗНЗЈ
ЃЈ1ЃЉBзАжУЕФУћГЦЃК______________
ЃЈ2ЃЉдкЩЯЪізАжУжаЃЌHФмПьЫйЁЂМђБужЦШЁЃЌзАжУжаашвЊЬэМгЕФЗДгІЪдМСЮЊ_________ ЁЃ
ЃЈ3ЃЉЮЊЬНОПИќКУЕФЪЕбщаЇЙћЃЌЛюЖЏаЁзщЭЌбЇВЩгУЩЯЪіCзАжУРДжЦШЁАБЦјЃЌдкПижЦЪЕбщЬѕМўЯрЭЌЕФЧщПіЯТЃЌЛёЕУЯТБэжаЪЕбщЪ§ОнЁЃ
ЗжЮіБэжаЪ§ОнЃЌФуШЯЮЊФФжжЗНАИжЦШЁАБЦјЕФаЇЙћзюКУ________ЃЈЬюађКХЃЉЃЌДгИУЗНАИбЁдёЕФдСЯЗжЮіжЦЦјаЇЙћКУЕФПЩФмдвђЪЧ________ЃЌ__________ЁЃ
ЪдМСзщКЯађКХ | ЙЬЬхЪдМС | NH 3 ЬхЛ§ЃЈmLЃЉ | |
a | 6.0gCa(OH) 2 Й§СП | 5.4gNH 4 Cl | 1344 |
b | 5.4g(NH 4 ) 2 SO 4 | 1364 | |
c | 6.0gNaOHЙ§СП | 5.4gNH 4 Cl | 1568 |
d | 5.4g(NH 4 ) 2 SO 4 | 1559 | |
e | 6.0gCaOЙ§СП | 5.4gNH 4 Cl | 1753 |
f | 5.4g(NH 4 ) 2 SO 4 | 1792 | |
II.ФЃФтЮВЦјДІРэ
ЛюЖЏаЁзщЭЌбЇбЁгУЩЯЪіВПЗжзАжУЃЌАДЯТСаЫГађСЌНгГЩФЃФтЮВЦјДІРэзАжУНјааЪЕбщЁЃ
ЃЈ1ЃЉЧыДгЩЯЪізАжУжабЁдёФуШЯЮЊКЯРэЕФНјааВЙГфЃЈЫљбЁзАжУВЛФмжиИДЃЉ_______ЁЃ
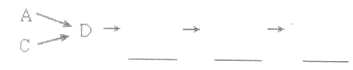
ЃЈ2ЃЉAжаЗДгІЕФРызгЗНГЬЪНЮЊ__________________________
ЃЈ3ЃЉDзАжУЕФзїгУгаЃК_____________ЁЂ____________ЁЂ_____________ЁЃ
ЃЈ4ЃЉDзАжУжаЕФвКЬхЛЙПЩЛЛГЩЃЈЬюађКХЃЉЁЃ
AЃЎH 2 O BЃЎCCl 4 CЃЎХЈH 2 SO 4 DЃЎCuSO 4 ШмвК
ЃЈ5ЃЉИУаЁзщЭЌбЇЫљЩшМЦЕФФЃФтЮВЦјДІРэзАжУжаЛЙДцдквЛДІУїЯдЕФШБЯнЪЧ___________.