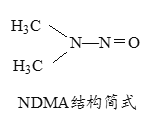
��Ŀ����
����Ŀ����1��������ȼ������285��8KJ/mol�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ ��
��2��1��6gҺ̬������N2H4��Һ̬H2O2ǡ����ȫ��Ӧ�������ֶԻ����Ѻõ����壬��ʱ�ų�75KJ����������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��3����֪Ba2++SO42��=BaSO4��s����H=-aKJ/mol����aΪ��֪����,��д��ϡ������ϡBa��OH��2��Һ��Ӧ���Ȼ�ѧ����ʽ ��
��4����֪2NH3��g��![]() N2��g��+3H2��g����H=+92��4KJ/mol����1molN2��g����3molH2�����ܱ������г�ַ�Ӧ����÷ų�������ʼ��С��92��4KJ���������ԭ�� ��
N2��g��+3H2��g����H=+92��4KJ/mol����1molN2��g����3molH2�����ܱ������г�ַ�Ӧ����÷ų�������ʼ��С��92��4KJ���������ԭ�� ��
��5������ϸ��ͼ��
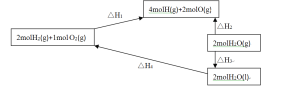
���ݸ�˹���ɣ���H4=������H1����H2����H3��ʾ��
���𰸡�
��1��H2��g��+0��5O2��g����H2O��l����H��-285��8kJ/mol��2��N2H4��l��+2H2O2��l����N2��g��+4H2O��g����H=-1500kJ/mol��3��H2SO4��aq��+Ba��OH��2��aq��=BaSO4��s��+2H2O��l����H=-��a+114��6��KJ/mol��4�����淴Ӧת����С��100%������N2��g��һ��С��1mol���ų�������һ��С��92��4kJ
��5����H4=��H2-��H1-��H3
��������
�����������1����һ�������£�1mol��ȼ����ȫȼ�������ȶ�������ʱ���ų���������ȼ���ȣ�������ȼ������285��8kJ/mol�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪH2��g��+1/2O2��g����H2O��l����H����285��8kJ/mol��
��2��1��6gҺ̬������N2H4��Һ̬H2O2ǡ����ȫ��Ӧ�������ֶԻ����Ѻõ����壬��ʱ�ų�75KJ������������ԭ���غ��֪���������ǵ�����ˮ�������÷�Ӧ���Ȼ�ѧ����ʽΪN2H4��l����2H2O2��l����N2��g��+4H2O��g����H=-1500kJ/mol����3����֪Ba2++SO42��=BaSO4��s����H=-akJ/mol����aΪ��֪����,�к�����57��3kJ/mol�����ϡ������ϡBa��OH��2��Һ��Ӧ���Ȼ�ѧ����ʽH2SO4��aq����Ba��OH��2��aq����BaSO4��s����2H2O��l����H������a+114��6��kJ/mol����4�����ڿ��淴Ӧת����С��100%������N2��g��һ��С��1mol�������ų�������һ��С��92��4kJ����5������ת����ϵͼ��ϸ�˹���ɿ�֪��H4����H2-��H1-��H3��