��Ŀ����
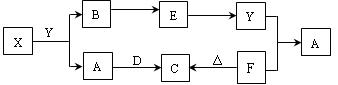
��10�֣���֪AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ�������³�ѹ��C��D��F��G��I������̬�������ʵ���A��B��������ˮ��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ����ش��������⣺
��1��д��B�ĵ���ʽ ��
��2��д��A��B��Ӧ�Ļ�ѧ����ʽ ��
��3��������� G���м�ѹ������������������ ��
G���м�ѹ������������������ ��
��4��д�����з�Ӧ�����ӷ���ʽ��
����A��Һ�м���M ��
����A��Һ�м������NaOH ��Һ�������� ��
��Һ�������� ��

��1��д��B�ĵ���ʽ ��
��2��д��A��B��Ӧ�Ļ�ѧ����ʽ ��
��3���������
 G���м�ѹ������������������ ��
G���м�ѹ������������������ ����4��д�����з�Ӧ�����ӷ���ʽ��
����A��Һ�м���M ��
����A��Һ�м������NaOH
 ��Һ�������� ��
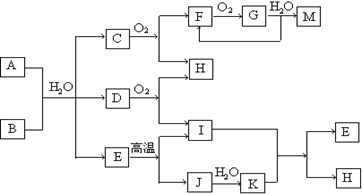
��Һ�������� ��A��NH4HCO3 B�� CaC2 C��NH3 D��C2H2
E��CaCO3 F��NO G��NO2 H��H2O I��CO2 J��CaO K��Ca(OH)2 M��HNO3
E��CaCO3 F��NO G��NO2 H��H2O I��CO2 J��CaO K��Ca(OH)2 M��HNO3
��

��ϰ��ϵ�д�
�����Ŀ


 ����һ�������£�2 L��H������0.5 L��G�������ϣ����û�����屻NaOH��Һǡ����ȫ���գ�ֻ����һ���Ρ���д���÷�Ӧ�������ܵĻ�ѧ��Ӧ����ʽ�� ��
����һ�������£�2 L��H������0.5 L��G�������ϣ����û�����屻NaOH��Һǡ����ȫ���գ�ֻ����һ���Ρ���д���÷�Ӧ�������ܵĻ�ѧ��Ӧ����ʽ�� ��