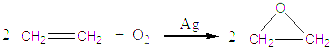��Ŀ����
��8�֣����Ȼ��غ��Ѱ׳��ĸ���Ʒ��������Ϊԭ����������ء���������狀������������ϣ� ԭ�ϵ��ۺ������ʽϸߡ�����Ҫ�������£�
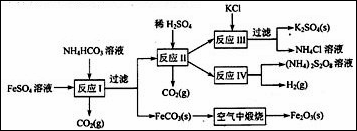
��1����ӦIǰ����FeSO4��Һ�м��� ������ĸ�����Գ�ȥ��Һ�е�Fe3����
��2����ӦI����Ʒ�Ӧ�¶ȵ�35�棬��Ŀ���� ��
��3����ҵ�����ϳ��ڷ�Ӧ��Ĺ����м���һ�����Ĵ����ܼ�����Ŀ���ǣ� ��
��4����Ӧ���������ڵ������(NH4)2S2O8 (���������)�����ʱ���ö��Ե缫������
�����ĵ缫��Ӧ�ɱ�ʾΪ�� ��

��1����ӦIǰ����FeSO4��Һ�м��� ������ĸ�����Գ�ȥ��Һ�е�Fe3����
| A��п�� | B����м | C��KI��Һ | D��H2 |
��3����ҵ�����ϳ��ڷ�Ӧ��Ĺ����м���һ�����Ĵ����ܼ�����Ŀ���ǣ� ��
��4����Ӧ���������ڵ������(NH4)2S2O8 (���������)�����ʱ���ö��Ե缫������
�����ĵ缫��Ӧ�ɱ�ʾΪ�� ��
��1��B
��2����ֹNH4HCO3�ֽ⣨�����Fe2+��ˮ�� ��
��3������K2SO4���ܽ�ȣ�������K2SO4����
��4��2SO42����2e����S2O82��
��2����ֹNH4HCO3�ֽ⣨�����Fe2+��ˮ�� ��
��3������K2SO4���ܽ�ȣ�������K2SO4����
��4��2SO42����2e����S2O82��
��1��Ϊ�˷�ֹFe2+������������Ӧ������ܷ�ֹFe2+���������ֲ������������ʵ����ʣ����Լ�����м��á�
��2���¶����ϸ����ٽ�Fe2+��ˮ�⣬Ҳ��ʹNH4HCO3�ֽ⣬���Է�Ӧ�¶�Ӧ��35�档
��3��ҪʹK2SO4������������Ҫ����������Һ�е��ܽ�ȣ�����K2SO4�����ӻ����
�ڴ����ܼ��е��ܽ��һ����С��
��4������ͼʾ֪�� (NH4)2SO4 (NH4)2S2O8 +H2
(NH4)2S2O8 +H2
H2Ӧ�������������������������ı仯��Ϊ��SO42����S2O82��
��2���¶����ϸ����ٽ�Fe2+��ˮ�⣬Ҳ��ʹNH4HCO3�ֽ⣬���Է�Ӧ�¶�Ӧ��35�档
��3��ҪʹK2SO4������������Ҫ����������Һ�е��ܽ�ȣ�����K2SO4�����ӻ����
�ڴ����ܼ��е��ܽ��һ����С��
��4������ͼʾ֪�� (NH4)2SO4
 (NH4)2S2O8 +H2
(NH4)2S2O8 +H2H2Ӧ�������������������������ı仯��Ϊ��SO42����S2O82��

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д� ����������ϵ�д�
����������ϵ�д�
�����Ŀ
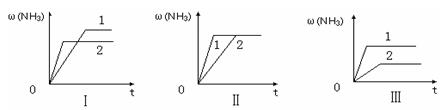
 3NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺
3NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺ H2(g) + CO(g) ��H =" +131.3" kJ ����S =" +133.7" J/K
H2(g) + CO(g) ��H =" +131.3" kJ ����S =" +133.7" J/K