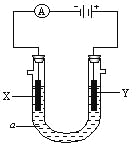
��Ŀ����
����Ŀ������(�Ҷ���)������ԭ���ͳ����������ڽ������⡢֯��Ư��ϡ��������һ���Ʊ�����(��2���ᾧˮ)�Ĺ����������ң�

�ش��������⣺
��1��CO��NaOH��һ�������ºϳɼ����ơ������Ƽ�������Ļ�ѧ��Ӧ����ʽ�ֱ�Ϊ��__________________��_______________��
��2�����Ʊ������������ι��˲��������˲���������Һ��___________��������________�����˲���������Һ��____________��____________��������_______��
��3�����չ�������������Ŀ����______________��
��4�����˽�������������ֱ���������ữ�Ʊ����ᡣ�÷�����ȱ���Dz�Ʒ���������к��е�������Ҫ��____________________��
��5���ᾧˮ�ϲ����Ʒ�Ĵ����ø�����ط��ⶨ��
���������Ʒ0.250 g����ˮ����0.0500 mol��L-1������KMnO4��Һ�ζ�����dz�ۺ�ɫ�����ʣ�����KMnO4��Һ15.00 mL����Ӧ�����ӷ���ʽΪ__________________��
��ʽ����ó�Ʒ�Ĵ���____________________________��
���𰸡�CO��NaOH![]() HCOONa 2HCOONa
HCOONa 2HCOONa![]() Na2C2O4��H2�� NaOH��Һ CaC2O4 H2C2O4��Һ H2SO4��Һ CaSO4 �ֱ�ѭ�������������ƺ�����(���ͳɱ�)����С��Ⱦ Na2SO4 5C2O42��+2MnO4��+16H��==2Mn2��+8H2O+10CO2��
Na2C2O4��H2�� NaOH��Һ CaC2O4 H2C2O4��Һ H2SO4��Һ CaSO4 �ֱ�ѭ�������������ƺ�����(���ͳɱ�)����С��Ⱦ Na2SO4 5C2O42��+2MnO4��+16H��==2Mn2��+8H2O+10CO2�� 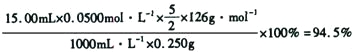
��������
��������ͼ֪��һ�������£�һ����̼���������Ʒ�Ӧ���ɼ����ƣ���Ӧ����ʽΪ��CO��NaOH![]() HCOONa�����������£��������������ɲ����ƣ���ԭ���غ��֪���������ɣ���Ӧ����ʽΪ2HCOONa
HCOONa�����������£��������������ɲ����ƣ���ԭ���غ��֪���������ɣ���Ӧ����ʽΪ2HCOONa![]() Na2C2O4��H2�������������������Ʒ�Ӧ�õ���������������ƣ���Һ��NaOH��Һ��Ũ����ѭ�����ã�����ΪCa C2O4������������ᷴӦ�õ������������ƣ�������ΪCaSO4����ҺBΪ��������Һ���پ����ᾧ������õ������ƾ��壬ĸҺ�к�������Ͳ��ᣬ��ѭ�����á�
Na2C2O4��H2�������������������Ʒ�Ӧ�õ���������������ƣ���Һ��NaOH��Һ��Ũ����ѭ�����ã�����ΪCa C2O4������������ᷴӦ�õ������������ƣ�������ΪCaSO4����ҺBΪ��������Һ���پ����ᾧ������õ������ƾ��壬ĸҺ�к�������Ͳ��ᣬ��ѭ�����á�
��1�����ݹ�������ͼ��֪��CO��NaOH��һ�������ºϳɼ����Ʒ���ʽΪCO��NaOH![]() HCOONa�������Ƽ��������ͬʱ���в��������ɣ���˷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ2HCOONa
HCOONa�������Ƽ��������ͬʱ���в��������ɣ���˷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ2HCOONa![]() Na2C2O4��H2����
Na2C2O4��H2����
��2�����ݷ�Ӧԭ�����ܽ����Լ�ʾ��ͼ��֪�����˲���������Һ��NaOH��Һ��������CaC2O4�����˲���������Һ��H2C2O4��Һ��H2SO4��Һ��������CaSO4��
��3�����ݹ��չ��̿�֪������������ѭ�������������ƣ�����������ѭ���������ᣬ�������ԭ�ϵ������ʣ��ֽ����˳ɱ�����С��Ⱦ��
��4�������������IJ���Ϊ�����ƣ�ֱ���������ữ�����ɲ���������ƣ����к��е�������Ҫ��Na2SO4��
��5���ڲⶨ�����У��������Ϊ������������Ϊ��ԭ������Ӧ�����ӷ���ʽ5C2O42- + 2MnO4- + 16H+= 2Mn2++ 8H2O + 10CO2�������ݷ���ʽ�ɵù�ϵʽ��
5H2C2O4��2H2O ~ 2KMnO4
5 2
n 0.05mol/L��15.0��10-3L
���n��H2C2O4��2H2O��=1.875��10-3mol
��m��H2C2O4��2H2O��=1.875��10-3mol��126g/mol=0.236g
���Գ�Ʒ�Ĵ�����=![]() ��100%=94.5%��
��100%=94.5%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�