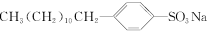题目内容
小明家责任田里的庄稼生长不茂盛,叶色淡绿、茎叶细小,同时还出现了不同程度的倒伏现象。请问:
(1)小明家的庄稼缺两种营养元素,它们是________(填“N”“P”“K”或“微量元素”)。
(2)某生产资料公司的化肥价格如下表:
化肥 | 尿素CO(NH2)2 | 碳铵NH4HCO3 | 磷矿粉Ca3(PO4)2 |
价格(元/吨) | 1 200 | 350 | 150 |
化肥 | 过磷酸钙Ca(H2PO4)2 | 氯化钾 | 硫酸钾 |
价格(元/吨) | 250 | 650 | 800 |
在考虑既经济实用又解决问题的条件下,你认为小明应购买的化肥是_____________(填化学式)。
(1)N、K (2)NH4HCO3、KCl
【解析】根据N、P、K营养元素对农作物生长的作用,易判断该同学家的庄稼
缺氮肥和钾肥。从生产资料公司提供的化肥价格看,花同样多的钱购买化肥,碳铵中氮元素的质量高于尿素中氮元素的质量,氯化钾中钾元素的质量高于硫酸钾中钾元素的质量。因此,该同学应购买NH4HCO3和KCl。

练习册系列答案
 亮点激活精编提优100分大试卷系列答案
亮点激活精编提优100分大试卷系列答案
相关题目