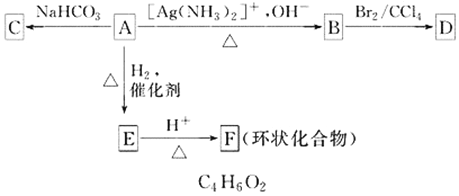
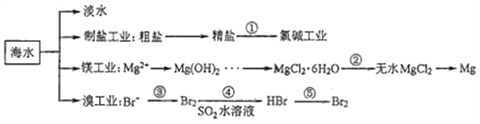
��Ŀ����
����Ŀ���ݱ������������أ�KH2PO4��������Ӧ�����ҹ����Ƶľ��ͼ����������������С����÷���ʯ����ѧʽΪCa5P3FO12)�Ʊ��������صĹ�����������ͼ��ʾ���������̲�����ʡ�ԣ���
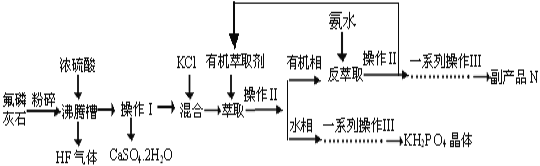
��֪��ȡ����Ҫ��Ӧԭ����KCl+H3PO4![]() KH2PO4+HCl�����У���Ӧ������HCl�������л���ȡ����
KH2PO4+HCl�����У���Ӧ������HCl�������л���ȡ����
��ش��������⣺
��1�������н�����ʯ�����Ŀ����__________________________________��
��2������ʹ�ö��������մɲ��ʵķ��ڲ۵���Ҫԭ����___________________���û�ѧ����ʽ��ʾ����
��3������ƷN�Ļ�ѧʽ��____________���ڵõ�KH2PO4�����һϵ�в�����������Ҫ����______________________________�����ˡ�ϴ�ӡ��������
��4������1000kg��������Ϊ50.4%�ķ���ʯ����ѧʽΪCa5P3FO12)����ȡ�������ؾ��壬�����Ϊ80%���������Ͽ�����KH2PO4������Ϊ_______kg��
��5����ⷨ�Ʊ�KH2PO4��װ����ͼ��ʾ���õ��װ���У�a ������_______�����������������������������������ĵ缫��Ӧʽ��______________________________________��

��6����ҵ�ϻ������÷���ʯ�뽹̿��ʯӢɰ��ϣ��ڵ�¯�м��ȵ�1500�����ɰ��ף�ͬʱ�ݳ�SiF4��CO,�÷�Ӧ�Ļ�ѧ����ʽΪ________________________________________��
���𰸡� �������ʯ��ϡ���ᷴӦ�ĽӴ����,�ӿ컯ѧ��Ӧ���� 4HF+SiO2�TSiF4��+2H2O NH4Cl ����Ũ������ȴ�ᾧ 326.4 kg ���� 2H����2e����H2�� 4Ca5P3FO12 +21SiO2+30C![]() 20CaSiO3+3P4+SiF4��+30CO��
20CaSiO3+3P4+SiF4��+30CO��
������������ʯ(��ѧʽΪCa5P3FO12)��������Ũ���ᣬ��Ӧ��������ᡢ����ơ�����ȣ������Ȼ��غ����л���ȡ����KCl+H3PO4![]() KH2PO4+HCl����Ӧ������HCl�������л���ȡ�����л����к����Ȼ��⣬���백ˮ��Ӧ�����Ȼ�泥���˸���Ʒ��ҪΪ�Ȼ�泥�ˮ���к���KH2PO4������һϵ�в����õ�KH2PO4���塣
KH2PO4+HCl����Ӧ������HCl�������л���ȡ�����л����к����Ȼ��⣬���백ˮ��Ӧ�����Ȼ�泥���˸���Ʒ��ҪΪ�Ȼ�泥�ˮ���к���KH2PO4������һϵ�в����õ�KH2PO4���塣
(1)�����н�����ʯ���飬�����������ʯ��ϡ���ᷴӦ�ĽӴ�������ӿ컯ѧ��Ӧ���ʣ��ʴ�Ϊ���������ʯ��ϡ���ᷴӦ�ĽӴ�������ӿ컯ѧ��Ӧ���ʣ�
(2)��������ͼ����Ӧ������������ᣬ������ܹ���������跴Ӧ����˲���ʹ�ö��������մɲ��ʵķ��ڲۣ��ʴ�Ϊ��4HF+SiO2�TSiF4��+2H2O��
(3)������������������ƷN�Ļ�ѧʽΪNH4Cl���ڵõ�KH2PO4�����һϵ�в�����Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ��ʴ�Ϊ��NH4Cl������Ũ������ȴ�ᾧ��
(4)1000kg��������Ϊ50.4%�ķ���ʯ(��ѧʽΪCa5P3FO12)�к���Ca5P3FO12������Ϊ504kg������PԪ���غ㣬�����Ͽ�����KH2PO4������Ϊ504kg��80%��![]() ��
��![]() =326.4 kg���ʴ�Ϊ��326.4 kg��
=326.4 kg���ʴ�Ϊ��326.4 kg��
(5)����ͼʾ��Ӧ����a������KH2PO4�����������b������a������a �������������������������ӷŵ������������缫��ӦʽΪ2H����2e����H2�����ʴ�Ϊ��������2H����2e����H2����
(6)�÷���ʯ�뽹̿��ʯӢɰ��ϣ��ڵ�¯�м��ȵ�1500�����ɰ��ף�ͬʱ�ݳ�SiF4��CO����Ӧ�Ļ�ѧ����ʽΪ4Ca5P3FO12 +21SiO2+30C![]() 20CaSiO3+3P4+SiF4��+30CO�����ʴ�Ϊ��4Ca5P3FO12 +21SiO2+30C
20CaSiO3+3P4+SiF4��+30CO�����ʴ�Ϊ��4Ca5P3FO12 +21SiO2+30C![]() 20CaSiO3+3P4+SiF4��+30CO����
20CaSiO3+3P4+SiF4��+30CO����

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����ҵ��ˮ�г�����һ���������Խ�ǿ��Cr2O72�������õζ�ԭ���ⶨCr2O72�������������£�
��������ȡ30.00 mL��ˮ����ƿ�У���������ϡ�����ữ��
����������������ĵ⻯����Һ��ַ�Ӧ��Cr2O72��+6I��+14H+ === 2Cr3++3I2+7H2O��
����������ƿ�е��뼸��ָʾ�����õζ�����ȡ0.1000 molL-1Na2S2O3��Һ���еζ������ݼ�¼���£���I2+2Na2S2O3 === 2NaI+Na2S4O6��
�ζ����� | Na2S2O3��Һ��ʼ����/mL | Na2S2O3��Һ�յ����/mL |
��һ�� | 1.02 | 19.03 |
�ڶ��� | 2.00 | 19.99 |
������ | 0. 20 | a |
��1����������ȡ30.00 mL��ˮѡ���������_____��
��2���������еμӵ�ָʾ��Ϊ_____���ζ��ﵽ�յ��ʵ��������____��
��3����������a �Ķ�����ͼ��ʾ������
�� a=_____��
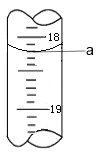
�� Cr2O72���ĺ���Ϊ____gL-1��
��4�����²�������ɷ�ˮ��Cr2O72�������ⶨֵƫ�ߵ���_____��
A. �ζ��յ����ʱ�����ӵζ��ܵĿ̶�
B. ʢװ����Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ
C. �ζ����յ����ʱ���ֵζ��ܼ��촦����һ����Һ
D. ��ȡNa2S2O3��Һ�ĵζ���������ˮϴ��δ�ñ�Һ��ϴ
����Ŀ�����и��������У�X������Y��Ӧ������Z��Ӧ����
Y | X | Z | |
�� | H2O��g�� | Fe | ZnSO4��Һ |
�� | KOH��Һ | Al | ϡ���� |
�� | Si | Cl2 | H2 |
�� | ���Ը��������Һ | Fe2����aq�� | Ư��Һ |
A.�٢�B.�٢�C.�ڢۢ�D.�ڢ�
����Ŀ����֪���ᾧ��(H2C2O4��2H2O)���۵�Ϊ 101�棬170��ֽ⡣����ѡ�õ�װ�ú�ҩƷ�ܴﵽʵ��Ŀ�ĵ��ǣ� ��
A | B | C | D |
|
|
|
|
��ȡSO2 | ��ȡNO2 | H2C2O4��2H2O�ֽ� | ���뱽���屽 |
A. AB. BC. CD. D