��Ŀ����
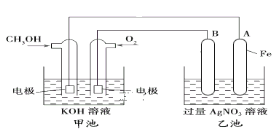
����Ŀ��ij��˾������һ���Լ״�Ϊԭ�ϣ���KOHΪ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡�����B�缫�ĵ缫����Ϊ̼����ͼ��һ���绯ѧ���̵�ʾ��ͼ��
����գ�
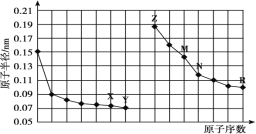
��1�����ʱ����ԭ��صĸ������Դ___��������
�������ĵ缫��ӦΪ____��
��2���ŵ�ʱ�������ĵ缫��ӦʽΪ____��
��3���ڴ˹���������ȫ��Ӧ���ҳ���A������������648 g����׳�������������O2____L(��״����)��
��4�����ڳ��³�ѹ�£�1gCH3OHȼ������CO2��Һ̬H2Oʱ����22.68kJ����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ____��
���𰸡��� 4OH��-4e��=2H2O��O2�� CH3OH-6e����8OH��=CO32-��6H2O 33.6 CH3OH(l)��![]() O2(g)=CO2(g)��2H2O(l) ��H����725.80kJ/mol
O2(g)=CO2(g)��2H2O(l) ��H����725.80kJ/mol
��������
��1�����ʱ��ԭ��ظ������Դ��������������������������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��4OH--4e-�T2H2O+O2����
�ʴ�Ϊ������4OH--4e-�T2H2O+O2����
��2���ŵ�ʱ���״�ʧ���Ӻ����������ӷ�Ӧ����̼������Ӻ�ˮ�����Ե缫��ӦʽΪ��CH3OH-6e-+8OH-�TCO32-+6H2O��
�ʴ�Ϊ��CH3OH-6e-+8OH-�TCO32-+6H2O��
��3���ҳ���B���������ӵõ��ӷ�����ԭ��Ӧ�����ҳ���B������������648g����׳�������������O2���= ��22.4L/mol=33.6L��
��22.4L/mol=33.6L��
�ʴ�Ϊ��33.6��
��4��n��CH3OH��=![]() mol������CO2��Һ̬H2Oʱ����22.68kJ����1molCH3OHȼ�շų�������Ϊ22.68kJ��32=725.80kJ����Ӧ���Ȼ�ѧ����ʽΪCH3OH(l)��
mol������CO2��Һ̬H2Oʱ����22.68kJ����1molCH3OHȼ�շų�������Ϊ22.68kJ��32=725.80kJ����Ӧ���Ȼ�ѧ����ʽΪCH3OH(l)��![]() O2(g)=CO2(g)��2H2O(l) ��H����725.80kJ/mol
O2(g)=CO2(g)��2H2O(l) ��H����725.80kJ/mol
�ʴ�Ϊ��CH3OH(l)��![]() O2(g)=CO2(g)��2H2O(l) ��H����725.80kJ/mol
O2(g)=CO2(g)��2H2O(l) ��H����725.80kJ/mol

����Ŀ����һ����ɫ�����ĩ����FeCl3��CaCO3��Na2SO4��KCl��Ba(NO3)2�еļ���������ɣ�ȡ��Ʒ��������ʵ�飨�������й����У��ܷ�Ӧ������֮��ķ�Ӧǡ����ȫ����

��1����������÷��뷽������___��Ҫ�Ӳ�������á���ɫ��Һ������ȡ�ܼ������÷��뷽������___��
��2��д��ʵ������з�����ѧ��Ӧ�����ӷ���ʽ��___����___��
��3�������ĩ��һ�������ڵ������ǣ��ѧʽ����ͬ��___������ȷ���Ƿ���ڵ�������___��
��4���������ĩ���ܵ���������±������Բ�������Ҳ�����ٲ��䣩��___
��� | ��ѧʽ |
�� | |
�� | |
��5�����һ��ʵ�飬��һ��ȷ����������ɣ�����ʵ�����������ͽ��ۡ�___