��Ŀ����
����Ŀ�����Ǻϳ����ᡢ��κ͵��ʵĻ���ԭ�ϡ���������е�Ҫ��ش��й����⣺
��1������ˮ��Һ�������ԣ���ԭ��Ϊ__________________________________________________(�����ӷ���ʽ��ʾ)��0.1 mol��L��1�İ�ˮ�м�������NH4Cl���壬��Һ��pH_______(ѡ��������������������)��������������������Һ��NH![]() ��Ũ��________(ѡ����������������С��)��
��Ũ��________(ѡ����������������С��)��
��2����֪����識��ȷֽ�ɵõ�N2O��H2O��250 ��ʱ������粒������ܱ������зֽ�ﵽƽ�⣬�÷ֽⷴӦ�Ļ�ѧ����ʽΪ��____________________________________________��ƽ�ⳣ������ʽΪ_____________________������1 mol�������ȫ�ֽ⣬����ת�Ƶĵ��ӵ����ʵ���Ϊ________mol��
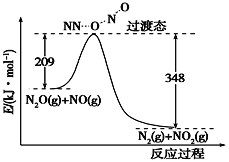
��3����N2O��NO��Ӧ����N2��NO2�������仯����ͼ��ʾ��������1 mol N2�����Ȼ�ѧ����ʽ����H��________kJ��mol��1��
���𰸡� NH3��H2O![]() NH
NH![]() ��OH�� ���� ���� NH4NO3
��OH�� ���� ���� NH4NO3![]() N2O��2H2O K=c(N2O)c2(H2O) 4 ��139
N2O��2H2O K=c(N2O)c2(H2O) 4 ��139
�������������������1��һˮ�ϰ�Ϊ�����ˮ��Һ�д��ڲ��ֵ��룬���������������ʹ��Һ�Լ��ԣ�����ʽΪ��NH3H2ONH4++OH-����ˮ�м�������NH4Cl���壬笠�Ũ������ƽ�����ƣ���������Ũ�ȼ�С��pHֵ���ͣ�������������������������������ӽ�����������������������ٽ���ˮ�ĵ��룬笠�Ũ�����ʴ�Ϊ��NH3H2ONH4++OH-�����ͣ�����
��2������立ֽ�����N2O��H2O���ﵽƽ�⣬˵��Ϊ���淴Ӧ����ѧ��Ӧ����ʽΪ��NH4NO3![]() N2O+2H2O��250��ʱ��ˮΪ����״̬����ƽ�ⳣ��K=c(N2O����c2(H2O����NH4NO3��NH4+��NԪ�ػ��ϼ�Ϊ-3�ۣ�NO3-�е�NԪ�صĻ��ϼ�Ϊ+5�ۣ���Ӧ��NԪ�صĻ��ϼ�Ϊ+1�ۣ��������з�Ӧ��NԪ����-3������Ϊ+1�ۣ��˷�Ӧ��ÿ�ֽ�1mol����泥�ת�Ƶ�����Ϊ4mol���ʴ�Ϊ��NH4NO3
N2O+2H2O��250��ʱ��ˮΪ����״̬����ƽ�ⳣ��K=c(N2O����c2(H2O����NH4NO3��NH4+��NԪ�ػ��ϼ�Ϊ-3�ۣ�NO3-�е�NԪ�صĻ��ϼ�Ϊ+5�ۣ���Ӧ��NԪ�صĻ��ϼ�Ϊ+1�ۣ��������з�Ӧ��NԪ����-3������Ϊ+1�ۣ��˷�Ӧ��ÿ�ֽ�1mol����泥�ת�Ƶ�����Ϊ4mol���ʴ�Ϊ��NH4NO3![]() N2O+2H2O��K=c(N2O����c2(H2O����4��
N2O+2H2O��K=c(N2O����c2(H2O����4��
��3����ͼ��֪���˷�Ӧ��Ӧ���������������������H=209-348=-139kJmol-1���ʴ�Ϊ��-139��

 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�����Ŀ����2L�ܱ������ڣ�800��ʱ��Ӧ2NO��g��+O2��g��=2NO2��g����ϵ�У�n��NO����ʱ��ı仯���±���
ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
n��NO����mol�� | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
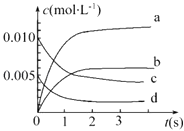
��1��������Ӧ�ڵ�5sʱ��NO��ת����Ϊ ��
��2����ͼ�б�ʾNO2�仯���ߵ��� �� ��O2��ʾ0��2s�ڸ÷�Ӧ��ƽ������v= ��
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬���� ��
a��v��NO2��=2v ��O2�� b��������ѹǿ���ֲ���
c��v����NO��=2v����O2�� d���������ܶȱ��ֲ��䣮