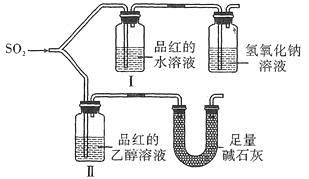
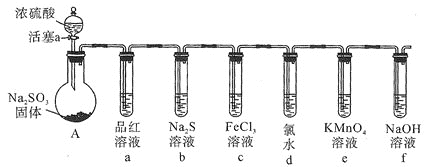
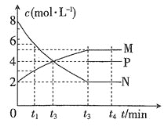
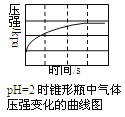
��Ŀ����
����Ŀ��X��Y��Z��W ������ԭ��������������Ķ�����Ԫ�أ�W �������������� X ��������������1����X��Y��ZΪͬһ����Ԫ�أ�X��Y��Z���һ�ֻ�����(ZXY)2 �Ľṹʽ��ͼ��ʾ������˵���������
![]()
A.������ WY �����õ����ȳ������
B.Y���������Ӧ��ˮ�������������
C.Y�ļ��⻯��ķе�һ������ Z
D.������(ZXY)2 ������ԭ�Ӿ����� 8 �����ȶ��ṹ
���𰸡�C
��������
X��Y��Z��W ������ԭ��������������Ķ�����Ԫ�أ�(ZXY)2 �Ľṹʽ�У�X����4�Ե��ӣ�ӦΪC��Si��W ��������������X��������������1������ԭ������W��X�����ԣ�Wֻ��ΪAl����XΪC��X��Y��ZΪͬһ����Ԫ�أ�(ZXY)2 �Ľṹʽ��Z����2�Ե��ӣ�Y����3�Ե��ӣ���ZӦΪO��YΪN����֮��X��Y��Z��W�ֱ�ΪC��N��O��Al�����ڴ˻����϶Ը�ѡ�������жϡ�
A. ���ݷ�����֪�������� WY��AlN��AlN��ԭ�Ӿ��壬�۵�ܸߣ����ȳ����Aѡ����ȷ��
B. Y���������Ӧ��ˮ�����У�HNO3��ǿ�ᣬHNO2�����ᣬBѡ����ȷ��
C. Y�ļ��⻯����NH3�������������壻Z�ļ��⻯����H2O����������Һ�壬���ԣ��е�H2O��NH3��Cѡ�����
D. ������(ZXY)2��(OCN)2������ʽ�ɱ�ʾΪ��![]() ������������ԭ��ͨ�����õ��Ӿ��ﵽ8�����ȶ��ṹ��Dѡ����ȷ��
������������ԭ��ͨ�����õ��Ӿ��ﵽ8�����ȶ��ṹ��Dѡ����ȷ��
��ѡC��

 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�