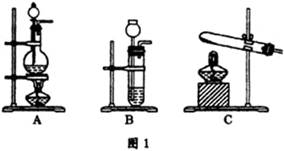
��Ŀ����
����ʧȥ��ǩ��CaCl2��AgNO3��HCl����Na2CO3��ƿ��Һ��Ϊ��ȷ��������Һ�ijɷ֣������DZ��ΪA��B��C��D����л�ѧʵ�顣ʵ���¼���£�
��������ʵ��������и�С�⣺
��1��A��B��C��D��ƿ��Һ�ֱ��ǣ��û�ѧʽ��ʾ�������ʣ�
A B C D
��2��д��B��D��Ӧ�����ӷ���ʽ
д��B��C��Ӧ�����ӷ���ʽ
| ʵ��˳�� | ʵ������ | ʵ������ |
| �� | A + B | ���������� |
| �� | B + D | ����ɫ��ζ����ų� |
| �� | C + B | �а�ɫ�������� |
| �� | A + D | �а�ɫ�������� |
��������ʵ��������и�С�⣺
��1��A��B��C��D��ƿ��Һ�ֱ��ǣ��û�ѧʽ��ʾ�������ʣ�
A B C D
��2��д��B��D��Ӧ�����ӷ���ʽ
д��B��C��Ӧ�����ӷ���ʽ
��1��CaCl2��HCl��AgNO3��Na2CO3
��2��CO32- +2H+ =CO2 ��+ H2O; Ag+ +Cl- ="AgCl" ��
��2��CO32- +2H+ =CO2 ��+ H2O; Ag+ +Cl- ="AgCl" ��
�����������1��B������3�����ʻ�ϣ�һ������Ϊ����ɫ���������һ������Ϊ�ɰ�ɫ�������ɣ�һ�������������ϵ�����ֻ��HCl��Ȼ����Ƴ�AΪCaCl2��CΪAgNO3��DΪNa2CO3��
��2��HCl��Na2CO3��Ӧ����NaCl��CO2��H2O�����ӷ���ʽΪ��CO32- +2H+ =CO2 ��+ H2O;HCl��AgNO3��Ӧ����AgCl��HNO3�����ӷ���ʽΪ��Ag+ +Cl- ="AgCl" ����

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
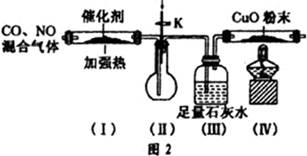
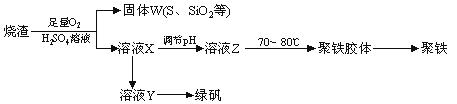
�����Ŀ
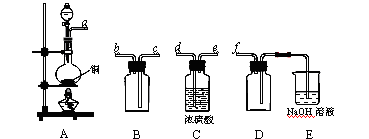
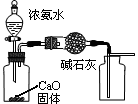
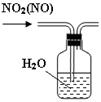
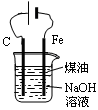
 ����1��ȡC�е�����������Ʒ���Թ��У��μ���������ˮ�������ܽ⣬Ȼ��������Һ�ֱ�����A��B�Թ��С�
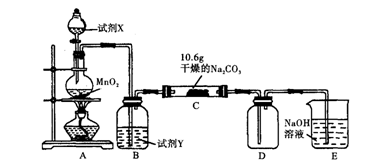
����1��ȡC�е�����������Ʒ���Թ��У��μ���������ˮ�������ܽ⣬Ȼ��������Һ�ֱ�����A��B�Թ��С�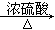 CO+H2O��ʵ��������ͼl��ʾ��װ�ã���ȡCO�����ѡ�õ�װ��Ϊ
CO+H2O��ʵ��������ͼl��ʾ��װ�ã���ȡCO�����ѡ�õ�װ��Ϊ