��Ŀ����
SO2��O2���ڴ��������ϽӴ�ʱ������Ӧ�ģ�����������������ķ����нӴ������Ӵ���������Ĺ�����SO2��ת����Ϊ90%����֪��101kPa ʱÿl mol SO2��������l mol SO3�ų�����98.3kJ���������Ȼ�ѧ����ʽ��ȷ���ǣ� ��A��SO2��g��+
 O2��g���TSO3��g������H=-98.3kJ?mol-1
O2��g���TSO3��g������H=-98.3kJ?mol-1B��2SO2��g��+O2��g���T2SO3��g������H=-176.94kJ?mol-1
C��SO2��g��+
 O2��g���TSO3��g������H=-88.47kJ?mol-1
O2��g���TSO3��g������H=-88.47kJ?mol-1D��2SO2��g��+O2��g���T2SO3��g������H=+196.6kJ?mol-1
����⣺����101kPa ʱÿl mol SO2��������l mol SO3�ų�����98.3kJ�������Ȼ�ѧ����ʽ��SO2��g��+
 O2��g���TSO3��g����H=-98.3kJ?mol-1��2SO2��g��+O2��g���T2SO3��g����H=-196.6kJ?mol-1����ѡ��A��
O2��g���TSO3��g����H=-98.3kJ?mol-1��2SO2��g��+O2��g���T2SO3��g����H=-196.6kJ?mol-1����ѡ��A��������������Ҫ�������Ȼ�ѧ����ʽ����д���Ȼ�ѧ����ʽ������֮�ȵ������ʵ���֮�ȣ�����Ϊ��+��������Ϊ��-����

 ���ݼ���ϵ�д�
���ݼ���ϵ�д�ʵʩ�Լ�����Դ�˷Ѻͽ��ͷ����ŷ�Ϊ�������ݵĽ��ܼ������ߣ���Ӧ��ȫ���������⡢������Դ��Լ�͡������Ѻ������ı�Ȼѡ������ҵ�ķ�չ������Ϲ��ҽ��ܼ��ŵ�����Ҫ����������֪ʶ����������������ѧ֪ʶ������������⣺
��1����֪ij��Ӧ��ƽ�����ʽΪ�� ������Ӧ�Ļ�ѧ��ӦΪ��_____________________________
������Ӧ�Ļ�ѧ��ӦΪ��_____________________________
��2������ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2��g��+CO��g��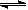 CH3OH��g������H=��90.8 kJ/mol
CH3OH��g������H=��90.8 kJ/mol
��2CH3OH��g��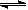 CH3OCH3��g��+H2O��g������H=��23.5 kJ/mol
CH3OCH3��g��+H2O��g������H=��23.5 kJ/mol
��CO��g��+ H2O��g��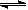 CO2��g��+H2��g������H=��41.3 kJ/mol
CO2��g��+H2��g������H=��41.3 kJ/mol
�ܷ�Ӧ��3H2��g��+3CO��g��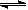 CH3OCH3��g��+CO2��g������H=__________ ��
CH3OCH3��g��+CO2��g������H=__________ ��
�����ѣ�CH3OCH3��ֱ����ȼ�ϵ�ؾ��������죬Ч�ʸߵ��ŵ㣬�������Ϊ���ԣ��õ�صĸ�����ӦΪ_____________________________��
��3��ú����ͨ��ͨ���о���ͬ�¶���ƽ�ⳣ���Խ������ʵ�����⡣��֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���ᷢ�����·�Ӧ��CO��g��+H2O��g�� ? H2��g��+CO2��g�����÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
? H2��g��+CO2��g�����÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
�¶�/�� | 400 | 500 | 800 |
ƽ�ⳣ��K | 9.94 | 9 | 1 |
�÷�Ӧ������Ӧ������_________��Ӧ������������������������������500��ʱ���У�����ʼʱCO��H2O����ʼŨ�Ⱦ�Ϊ0.020mol/L���ڸ������£�CO��ƽ��ת����Ϊ��________��
��4���Ӱ����������������ᣬ�˹������漰���������NO��NO2��N2O4�ȡ��Է�Ӧ? N2O4��g��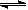 2NO2��g��? ��H>0�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����___________��
2NO2��g��? ��H>0�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����___________��

A��A��C����ķ�Ӧ���ʣ�A��C
B��A��C�����������ɫ��A�Cdz
C��B��C����������ƽ����Է���������B��C
D����״̬B��״̬A�������ü��ȵķ���
E��A��C����Ļ�ѧƽ�ⳣ����A��C
��5��NO2���ð�ˮ��������NH4NO3 ��25��ʱ����m mol NH4NO3����ˮ����Һ�����ԣ������Һ�μ�n L��ˮ����Һ�����ԣ���μӰ�ˮ�Ĺ�����ˮ�ĵ���ƽ�⽫______�������������������������������ƶ������μӰ�ˮ��Ũ��Ϊ_______mol��L-1����NH3��H2O�ĵ���ƽ�ⳣ��ȡKb=2X10-5 mol��L-1��
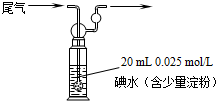
��6��ij���е�λ����ԭ���ԭ������SO2��O2���Ʊ����ᣬװ������ͼ���缫Ϊ��IJ������������壬ͬʱҲ��ʹ������������Һ��ֽӴ���

����Һ��H+���ƶ�������______ ����______��������A��B��ʾ��
��B�缫�ĵ缫��ӦʽΪ__________________________��
 ��2009?̩��ģ�⣩���������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ�
��2009?̩��ģ�⣩���������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ�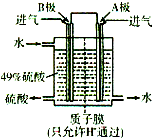 ��2012?Ϋ��һģ��������Fe2S������SΪ-1�ۣ��ǹ�ҵ�����������Ҫԭ�ϣ�FeS2�ڸ�������������Ӧ��3FeS2+8O2
��2012?Ϋ��һģ��������Fe2S������SΪ-1�ۣ��ǹ�ҵ�����������Ҫԭ�ϣ�FeS2�ڸ�������������Ӧ��3FeS2+8O2