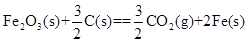��Ŀ����
����Ϊ�ı�δ�������ʮ���¿Ƽ�֮һ��ȼ�ϵ�ؾ�������Ⱦ������������Ч�ʵ��ص㣮��ͼΪ����ȼ�ϵ�صĽṹʾ��ͼ���������ҺΪKOH��Һ���缫����Ϊ���ɶ��ʯī�����������������ֱ��������ϵش�����������ͨ��ȼ�ϵ��ʱ������ڱպϻ�·�в��ϵز����������Իش��������⣺
��1�� д������ȼ�ϵ�ع���ʱ�����缫��Ӧ����ʽ��
___________ ��
��2�����������ȼ�ϵ��ÿת��0.1mol���ӣ����ı�״����_______L������
��3�� ������ȼ�ϵ�ظĽ�Ϊֱ���Լ��������Ϊԭ�Ͻ��й���ʱ��������ӦʽΪ_______�� ��������ӷ�Ӧ����ʽΪ___________________________��
��1��2H2O��O2��4e��=4OH�� ��2�֣�
��2��0.56L��2�֣�
��3��CH4��10OH����8e��=CO32-��7H2O ��2�֣�
CH4��2O2��2OH��=CO32-��3H2O��2�֣�
���������������Ϊ����ȼ�ϵ�صĵ������ҺΪ���ԣ�����������ӦʽΪ2H2O��O2��4e��=4OH������ÿת��1mol���ӣ����ı�״��������5.6L������ת��0.1mol����ʱ�����ı�״��������0.56L,���Լ���Ϊȼ��ʱ������Ϊ������Ӧ��缫��ӦʽΪCH4��10OH����8e��=CO32-��7H2O��������ӦʽΪ2O2+4H2O+8e-=8OH-,�����缫��Ӧʽ�ϲ�Ϊ��ص��ܷ�ӦCH4��2O2��2OH��=CO32-��3H2O��
���㣺ԭ��ط�Ӧ����ʽ����д����ؼ��㡣

�������������ɫ�ͳ����µ�Ksp���±���ʾ��
| | Cu(OH)2 | CuOH | CuCl | Cu2O |
| ��ɫ | ��ɫ | ��ɫ | ��ɫ | ש��ɫ |
| Ksp(25 ��) | 1��6��10��19 | 1��0��10��14 | 1��2��10��6 | �� |
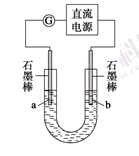
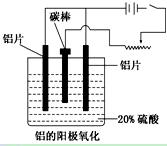
ij�о���ѧϰС��Ե��ʳ��ˮ����������̽����
ʵ���װ����ͼ��ʾ����ͨ��Դ����a��b�缫�Ͼ������ݲ�����
��1���������е������ӷ�Ӧ����ʽΪ_________________________________________��
��2��Ϊ��ȷ����Դ���������������в���һ����֮��Ч�������� ������
A���۲����������������ɫ
B����U�ι����˷ֱ�������η�̪��Һ
C����ȼ�ŵ�ľ������U�ιܿ�
D����U�ιܿ���һ��ʪ��ĵ���KI��ֽ
ʵ�����������װ�õ�ʯī������ͭ������ֱ����Դ���е�⣬װ����ͼ��ʾ��

�۲쵽������������ʾ��
�ٿ�ʼ�������������Һ�����µ�ͭ�������䰵��
��5 min��b��������ʼ���ְ�ɫ�������������࣬����a����ɢ��
��10 min�����a���İ�ɫ������ʼ��ɺ�ɫ��
��12 min��b�������İ�ɫ������ʼ��ɻ�ɫ��Ȼ����ɳȻ�ɫ��
��a��һֱ�д������ݲ�����
��ֹͣ��⣬��U�ι�������Һ����һ��ʱ����ϲ���Һ����ɫ��û�г�����ɫ���²����ȫ����ש��ɫ��
��3�� a�������ĵ缫��Ӧ����ʽΪ________________________________________________________��
��4�� ���5 min��b�������ĵ缫��Ӧ����ʽΪ___________________________________________��
��5��12 min��b���������ֵijȻ�ɫ�����ijɷ���������������ԭ����___________________________________________________________________________________��
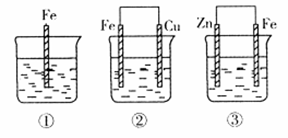
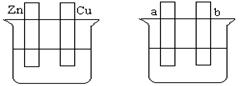
��10�֣�ij��ѧ��ȤС���ͬѧΪ��̽�����缫�ڵ���е�����,��Ʋ�����������һϵ��ʵ��,ʵ������¼����:
| ��� | �缫���� | �������Һ | ������ָ��ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��C(ʯī) | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | ����������Һ | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
��1��ʵ��1��2��Al�����ĵ缫(������) (���ͬ������ͬ��)��
��2��ʵ��3�и�����Ӧʽ���� ���ܷ�Ӧ�����ӷ���ʽ�� �� ��
��3��ʵ��4�������������������缫��Ӧʽ���� ��
��4������ʵ��5�е�����ָ��ƫ������ԭ�� ��
 4Al��3O2��
4Al��3O2�� Fe(OH)2+Ni(OH)2
Fe(OH)2+Ni(OH)2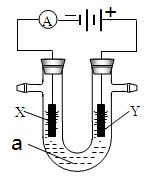
 ��-2830kJ��mol-1
��-2830kJ��mol-1