��Ŀ����
A��B��C��D��E����Ԫ�أ����ǵ����������ε���������С��18��AԪ��ԭ�Ӻ���ֻ��1�����ӣ�BԪ��ԭ�Ӻ�CԪ��ԭ������������֮��Ϊ2��BԪ��ԭ��������ϵĵ�����Ϊ������������2����B��C��D����Ԫ�ؿ����γɻ�ѧʽΪD2BC3�Ļ��������ɫ��ӦΪ��ɫ��0.5mol EԪ�صĵ������������ᷴӦ��9.03��1023�����ӷ���ת�ƣ���E��C�Ļ������У�E������ռ52.94%������֪E��ԭ�Ӻ�����14�����ӣ�
��1����������Ԫ�طֱ��ǣ�
A______��B______��C______��
��2������Dԭ�ӽṹʾ��ͼ��E�����ӽṹʾ��ͼ______��______��
��3��д��A2C��BC2��D2C2�ĵ���ʽ______��______��______��
��4��д��D2C2��A2C��Ӧ�Ļ�ѧ����ʽ______��
��1����������Ԫ�طֱ��ǣ�
A______��B______��C______��
��2������Dԭ�ӽṹʾ��ͼ��E�����ӽṹʾ��ͼ______��______��
��3��д��A2C��BC2��D2C2�ĵ���ʽ______��______��______��
��4��д��D2C2��A2C��Ӧ�Ļ�ѧ����ʽ______��
��1��AԪ��ԭ�Ӻ���ֻ��1�����ӣ���AΪH��BԪ��ԭ��������ϵĵ�����Ϊ������������2������BΪC��BԪ��ԭ�Ӻ�CԪ��ԭ������������֮��Ϊ2�����������ε�������CΪO���ʴ�Ϊ����H�� ̼C����O��
��2��D2BC3�Ļ��������ɫ��ӦΪ��ɫ����DΪNa��0.5 mol EԪ�صĵ������������ᷴӦ��9.03��1023�����ӷ���ת�ƣ���EΪ+3�ۣ���E��C�Ļ�������E������ռ52.94%������֪E��ԭ�Ӻ�����14�����ӣ���EΪAl���ʴ�Ϊ��Na
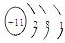
Al3+
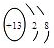
��
��3��A2CΪH2O��BC2ΪCO2��D2C2ΪNa2O2���ʴ�Ϊ��
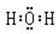
��
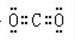
��
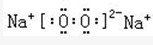
��
��4��D2C2��A2C��Ӧ�Ļ�ѧ����ʽ��2Na2O2+2H2O=4NaOH+O2�����ʴ�Ϊ2Na2O2+2H2O=4NaOH+O2����
��2��D2BC3�Ļ��������ɫ��ӦΪ��ɫ����DΪNa��0.5 mol EԪ�صĵ������������ᷴӦ��9.03��1023�����ӷ���ת�ƣ���EΪ+3�ۣ���E��C�Ļ�������E������ռ52.94%������֪E��ԭ�Ӻ�����14�����ӣ���EΪAl���ʴ�Ϊ��Na
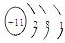
Al3+
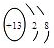
��
��3��A2CΪH2O��BC2ΪCO2��D2C2ΪNa2O2���ʴ�Ϊ��
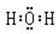
��
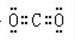
��

��
��4��D2C2��A2C��Ӧ�Ļ�ѧ����ʽ��2Na2O2+2H2O=4NaOH+O2�����ʴ�Ϊ2Na2O2+2H2O=4NaOH+O2����

��ϰ��ϵ�д�
�����Ŀ






 Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������
Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������