��Ŀ����
����Ŀ���п�Ժ������ѧ�����о�����һ�����³ɹ�ʵ���˼����Ч������ϩ�������ڴ����������⣬�������о����ɻ�ż����Ӧ������ϩ����ͼ��ʾ��

��1���ִ�ʯ�ͻ�������Ag����������ʵ����ϩ�������Ʊ�X������ʽC2H4O������˫�������÷�Ӧ�����������ԭ�Ӿ��ã���Ӧ�Ļ�ѧ����ʽ��______________���л�����д�ṹ��ʽ����
��2����֪������ʵ�ȼ�������ϱ���д�������Ʊ���ϩ���Ȼ�ѧ����ʽ_____________��
��3����400 ��ʱ�����ʼ���1 L�ĺ�ѹ��Ӧ���г���1 molCH4������������Ӧ�����ƽ����������C2H4���������Ϊ20.0%����
���ڸ��¶��£���ƽ�ⳣ��K��________��
�����������ͨ�����ˮ���������μӷ�Ӧ������400�棩��C2H4�IJ��ʽ�________��ѡ��������������С��������������ȷ��������������_____________��
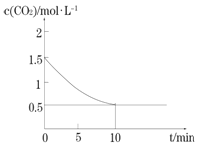
������������̶�����ͬѹǿ�¿ɵñ仯����ͼ����ѹǿ�Ĺ�ϵ��__________��

��ʵ���Ʊ�C2H4ʱ��ͨ�����ڸ���Ӧ��2CH4(g) ��C2H6(g)��H2(g)����Ӧ����CH4��ʼ�����䣬��ͬ�¶���C2H6��C2H4������������¶ȵĹ�ϵ������ͼ��

A����200 ��ʱ����������������ϩ�����Ҫԭ�������_____________��
B��400��ʱ��C2H4��C2H6����������ֱ�Ϊ20.0%��6.0%������ϵ��CH4�����������_________��
���𰸡�2CH2 = CH2 + O2![]() 2
2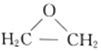 2CH4��g��
2CH4��g��![]() C2H4��g��+2H2��g����H=+202.0kJ/mol 0.20mol/L ���� �÷�ӦΪ���������������ȷ�Ӧ��ͨ�����ˮ�����൱�ڼ��ȣ�ͬʱͨ��ˮ��������������������൱�ڼ�Сѹǿ��ƽ������ƣ��������� p1��p2 ��200��ʱ��������������ʱ���ϩ�Ŀ� 28%
C2H4��g��+2H2��g����H=+202.0kJ/mol 0.20mol/L ���� �÷�ӦΪ���������������ȷ�Ӧ��ͨ�����ˮ�����൱�ڼ��ȣ�ͬʱͨ��ˮ��������������������൱�ڼ�Сѹǿ��ƽ������ƣ��������� p1��p2 ��200��ʱ��������������ʱ���ϩ�Ŀ� 28%
��������
(1)X�ķ���ʽC2H4O������˫�����жϳ�X�Ľṹ��ʽ����Ӧ�����������ԭ�Ӿ��ã��ݴ���д��Ӧ�Ļ�ѧ����ʽ��
(2)���ݱ�����������дH2��CH4��C2H4ȼ���ȵ��Ȼ�ѧ����ʽ��Ȼ����ݸ�˹���ɼ�������Ʊ���ϩ�ķ�Ӧ�ȣ�
(3)�ٸ�������ʽ���ƽ����������C2H4���������Ϊ20.0%���㣻
��ͨ�����ˮ�������൱�ڼ��Ⱥͼ�Сѹǿ������(2)�еķ�Ӧ�����ƽ���Ӱ�����ط������
������������̶������ݷ�Ӧ���������ѹǿ��ƽ���Ӱ������жϣ�
��A.����ͼ���Ϸ�Ӧ���ʽ��B.����������������ʵ���Ϊx�������ƽ��ʱC2H4��C2H6�����������ʵ������ټ����������������
(1)�ִ�ʯ�ͻ�������Ag����������ʵ����ϩ�������Ʊ�X(����ʽC2H4O������˫��)��XΪ ���÷�Ӧ�����������ԭ�Ӿ��ã���Ӧ�Ļ�ѧ����ʽΪ2CH2 = CH2 + O2
���÷�Ӧ�����������ԭ�Ӿ��ã���Ӧ�Ļ�ѧ����ʽΪ2CH2 = CH2 + O2![]() 2
2 ���ʴ�Ϊ��2CH2 = CH2 + O2
���ʴ�Ϊ��2CH2 = CH2 + O2![]() 2
2 ��
��
(2)���ݱ����������У���H2(g)+![]() O2(g)�TH2O(l)��H1=-285.8kJ/mol����CH4(g)+2O2(g)��CO2(g)+2H2O(l)��H2=-890.3kJ/mol����C2H4(g)+3O2(g)��2CO2(g)+2H2O(l)��H3=-1411.5kJ/mol�������Ʊ���ϩ�Ļ�ѧ����ʽΪ��2CH4(g)��C2H4(g)+2H2(g)�����ݸ�˹���ɣ�������2-��-����2�õ���2CH4(g)��C2H4(g)+2H2(g) ��H=2��H2-��H3-2��H1 =+202.5kJ/mol���ʴ�Ϊ��2CH4(g)��C2H4(g)+2H2(g)��H=+202.5 kJ/mol��
O2(g)�TH2O(l)��H1=-285.8kJ/mol����CH4(g)+2O2(g)��CO2(g)+2H2O(l)��H2=-890.3kJ/mol����C2H4(g)+3O2(g)��2CO2(g)+2H2O(l)��H3=-1411.5kJ/mol�������Ʊ���ϩ�Ļ�ѧ����ʽΪ��2CH4(g)��C2H4(g)+2H2(g)�����ݸ�˹���ɣ�������2-��-����2�õ���2CH4(g)��C2H4(g)+2H2(g) ��H=2��H2-��H3-2��H1 =+202.5kJ/mol���ʴ�Ϊ��2CH4(g)��C2H4(g)+2H2(g)��H=+202.5 kJ/mol��
(3)��400��ʱ����1L�ĺ��ݷ�Ӧ���г���1mol CH4������������Ӧ�����ƽ����������C2H4���������Ϊ20.0%��
2CH4(g)C2H4(g)+2H2(g)
��ʼ(mol) 1 0 0
ת��(mol) 2x x 2x
ƽ��(mol) 1-2x x 2x
������![]() ��20.0%����ã�x=0.25��ƽ�����������=
��20.0%����ã�x=0.25��ƽ�����������=![]() ��1L=1.25L�����Ի�ѧƽ�ⳣ��ΪK=
��1L=1.25L�����Ի�ѧƽ�ⳣ��ΪK=![]() =
= 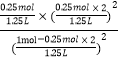 =0.20mol/L���ʴ�Ϊ��0.20mol/L��
=0.20mol/L���ʴ�Ϊ��0.20mol/L��
��2CH4(g)��C2H4(g)+2H2(g)��H=+202.5 kJ/mol����ӦΪ���������������ȷ�Ӧ��ͨ�����ˮ����(���μӷ�Ӧ������400��)�൱�ڼ��ȣ�ƽ�����ƣ���������ͬʱͨ��ˮ��������������������൱�ڼ�Сѹǿ��ƽ�����ƣ�����Ҳ�������C2H4�IJ��ʽ����ʴ�Ϊ�����÷�ӦΪ���������������ȷ�Ӧ��ͨ�����ˮ�����൱�ڼ��ȣ�ͬʱͨ��ˮ��������������������൱�ڼ�Сѹǿ��ƽ������ƣ���������
������������̶���2CH4(g)��C2H4(g)+2H2(g)����ӦΪ�������������ķ�Ӧ���¶���ͬʱ��ѹǿ�������ڷ�Ӧ������У�CH4��ƽ��ת���ʽ��ͣ����p1��p2���ʴ�Ϊ��p1��p2��
��A.����ͼ��200��ʱ����������������ϩ�࣬����Ϊ��������ķ�Ӧ���ʽϿ죬�ʴ�Ϊ����200��ʱ��������������ʱ���ϩ�Ŀ죻
B.����������������ʵ���Ϊx����C2H4Ϊ0.2x��C2H6Ϊ0.06x������2CH4(g)��C2H4(g)+2H2(g)��2CH4(g) ��C2H6(g)��H2(g)��֪�����ɵ�����Ϊ0.4x+0.06x=0.46x�����еļ���Ϊx-(0.2x+0.06x+0.46x)=0.28x�������ϵ��CH4���������=![]() ��100%=28%���ʴ�Ϊ��28%��
��100%=28%���ʴ�Ϊ��28%��