��Ŀ����
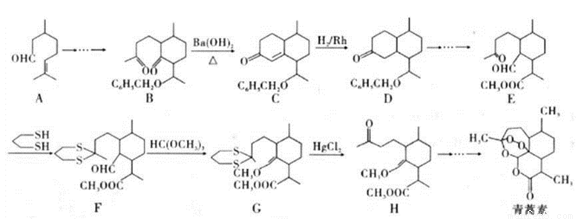
�л���M���л��ϳɵ���Ҫ�м��壬�Ʊ�M��һ�ֺϳ�·�����£����ַ�Ӧ�������Լ�����ȥ��
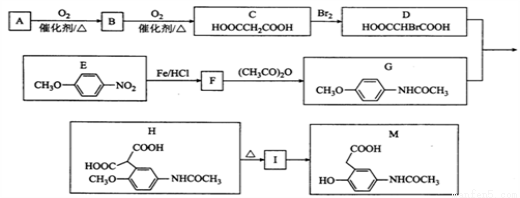
��֪�� ��A���ܶ�����ͬ������H2�ܶȵ�38��������ӵĺ˴Ź�����������3��壻
��
��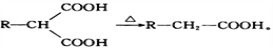
��ش��������⣺
��1��B�Ļ�ѧ����Ϊ______________��A�й����ŵĵ���ʽΪ________________��
��2��C�й����ԭ�������________����I�Ľṹ��ʽΪ_____________________��
��3��F��G�Ļ�ѧ����ʽΪ________________________________________________��
��4��M�����ܷ����ķ�ӦΪ_______________����ѡ����ĸ��
A.�ӳɷ�Ӧ B.������Ӧ C.ȡ����Ӧ D.��ȥ��Ӧ
��5��ͬʱ��������������E��ͬ���칹����_________�֡�
������FeCl3��Һ������ɫ��Ӧ ������NaHCO3��Ӧ �ۺ��С�����NH2
��6�����������ϳ�·�ߣ��� 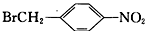 Ϊԭ�ϣ����Լ���ѡ��������Ʊ�
Ϊԭ�ϣ����Լ���ѡ��������Ʊ�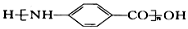 �ĺϳ�·�ߣ�_______________________________________________��
�ĺϳ�·�ߣ�_______________________________________________��
 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�����ʵ�鷽���У����ܴﵽʵ��Ŀ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | ����CH3CH2Br��NaOH��Һ���Ƿ���ˮ�� | ��CH3CH2Br��NaOH��Һ���ȡ���ȴ��ȡ���ϲ�ˮ��Һ����ϡHNO3�ữ������AgNO3��Һ���۲��Ƿ��������ɫ���� |
B | ����Fe(NO3)2�����Ƿ����������� | ��Fe(NO3)2��Ʒ����ϡH2SO4�μ�KSCN��Һ���۲���Һ�Ƿ��� |
C | ��֤Br2��������ǿ��I2 | ��������ˮ����KI��Һ�У��ټ���CCl4�������ã��ɹ۲쵽�²�Һ�����ɫ |
D | ��֤AgI���ܽ��С��AgCl | ��NaIŨ��Һ����AgCl����Һ�У����ɹ۲쵽�����ɰ�ɫ��Ϊ��ɫ |
A. A B. B C. C D. D




 CH2CH2CHO)��·������ͼ��_______________________�����Լ���ѡ���ϳ�·������ͼʾ�����£�
CH2CH2CHO)��·������ͼ��_______________________�����Լ���ѡ���ϳ�·������ͼʾ�����£�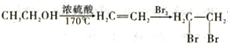 ��
��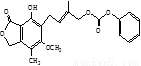

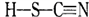 )��B-��������(
)��B-��������(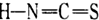 )������Ϊ��_________�������۵�ϸߵ���___________ (�����)��ԭ����________________________________��
)������Ϊ��_________�������۵�ϸߵ���___________ (�����)��ԭ����________________________________��