��Ŀ����
������������������ݣ�H2O18.0��CaO56.0��CaO272.0��
����������һ�ְ�ȫ���������ͨ�����в���CaO���Ҵ����������ȵĽᾧˮ��Ϊ����ij����������Ʒ����ɽ���������ʵ�顣
�ٳ�ȡ0.270g��Ʒ������ʹ֮��ȫ�ֽ⣬����CaO��O2��H2O���õ���O2�ڱ�״�������Ϊ33.6mL��
����ȡ0.120g��Ʒ������ϡ���ᣬ������У�ʹ���ɵ�H2O2��ȫ�ֽ⡣Ȼ����Һ�е�Ca2+��ȫת����CaC2O4������������ϴ�Ӻ����������ȵ�ϡ���ᣬ��0.0200mol��L-1KMnO4��Һ�ζ�������ȥ31.0mLKMnO4��Һ����ѧ����ʽ���£�
5CaC2O4+2KMnO4+8H2SO4==K2SO4+2MnSO4+5CaSO4+10CO2��+8H2O
��1��д��CaO2���ȷֽ�Ļ�ѧ����ʽ��
��2��������Ʒ��CaO2������������
��3��������Ʒ��CaO2��xH2O��xֵ��
����������һ�ְ�ȫ���������ͨ�����в���CaO���Ҵ����������ȵĽᾧˮ��Ϊ����ij����������Ʒ����ɽ���������ʵ�顣
�ٳ�ȡ0.270g��Ʒ������ʹ֮��ȫ�ֽ⣬����CaO��O2��H2O���õ���O2�ڱ�״�������Ϊ33.6mL��
����ȡ0.120g��Ʒ������ϡ���ᣬ������У�ʹ���ɵ�H2O2��ȫ�ֽ⡣Ȼ����Һ�е�Ca2+��ȫת����CaC2O4������������ϴ�Ӻ����������ȵ�ϡ���ᣬ��0.0200mol��L-1KMnO4��Һ�ζ�������ȥ31.0mLKMnO4��Һ����ѧ����ʽ���£�
5CaC2O4+2KMnO4+8H2SO4==K2SO4+2MnSO4+5CaSO4+10CO2��+8H2O
��1��д��CaO2���ȷֽ�Ļ�ѧ����ʽ��
��2��������Ʒ��CaO2������������
��3��������Ʒ��CaO2��xH2O��xֵ��
��1��2CaO2 2CaO+O2��
2CaO+O2��
��2��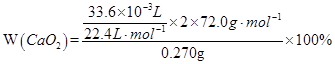 =80.0��
=80.0��
��3��n��CaC2O4��=n��Ca2+��=31.0��10-3L��0.0200mol��L-1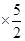 =0.00155mol
=0.00155mol
����CaO2������n��Ca2+��=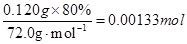
m��CaO��=��0.00155mol-0.00133mol����56.0g��mol-1=0.012g
m��H2O��=0.120g-0.120g��80.0��-0.012g=0.012g
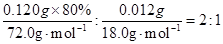
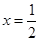
 2CaO+O2��
2CaO+O2����2��
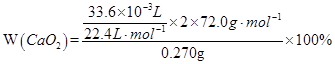 =80.0��
=80.0����3��n��CaC2O4��=n��Ca2+��=31.0��10-3L��0.0200mol��L-1
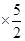 =0.00155mol
=0.00155mol����CaO2������n��Ca2+��=
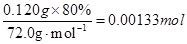
m��CaO��=��0.00155mol-0.00133mol����56.0g��mol-1=0.012g
m��H2O��=0.120g-0.120g��80.0��-0.012g=0.012g
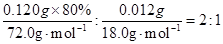
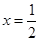
������������ڣ�1���ʣ���CaO2==CaO+O2����CaO2==Ca+O2����2CaO2+H2O==2CaO+O2+H2O����2CaO2��nH2O==2CaO+O2+2nH2O��n=1��2��3��������ֵ����
�ڣ�2���ʣ���40������62������[
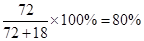 ����Դ:
����Դ:�ڣ�3���ʣ��٣�72+18x����0.120=1����1.55��10-3������72��M��CaO2��xH2O��=80����M=90��x=1��
�����⡿�ڣ�1���ʣ�������Ϣ�٣���д��CaO2���ȷֽ�Ļ�ѧ����ʽ��
2CaO2
 CaO+O2����������������������δ��ƽ���¡�������������δ������ʾ��Ϣ���¡������������ڶ�д��ˮ�ķ���ʽ���¡�������������δͨ��ȫ�����������CaO2��xH2Oд���ж�ֵ��ˮ�����ʧ�ڣ�2���ʣ�������Ϣ���У�O2�ڱ�״���µ��������������ʵ���Ϊ33.6mL��22400mL��mol-1=1.50��10-3mol��
CaO+O2����������������������δ��ƽ���¡�������������δ������ʾ��Ϣ���¡������������ڶ�д��ˮ�ķ���ʽ���¡�������������δͨ��ȫ�����������CaO2��xH2Oд���ж�ֵ��ˮ�����ʧ�ڣ�2���ʣ�������Ϣ���У�O2�ڱ�״���µ��������������ʵ���Ϊ33.6mL��22400mL��mol-1=1.50��10-3mol�����ݹ�ϵʽ2CaO2��O2�������CaO2���ʵ���Ϊ2��1.50��10-3mol��CaO2����Ϊ2��1.50��10-3mol��72.0g��mol-1
��Ʒ��CaO2����������Ϊ��
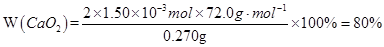
���֢������������ڻ�ѧ����ʽδ��ƽ����ϵʽδ�Ҷԣ�CaO2��O2��������ʧ���֢���������������CaO2��Ħ������72.0g��mol-1��CaO��Ħ������56.0g��mol-1����ʽ�м��㣬����ʧ���֢�������������δ���������ݣ�ֱ����CaO2��H2O��Ħ�������������㣬��Ȼ����������80��������������ݴ���
�ڣ�3���ʣ�������Ϣ�ڣ������CaC2O4���ʵ�������Ca2+�����ʵ����������ù�ϵʽΪ��
5CaC2O4��2KMnO4
KMnO4�����ʵ���Ϊ0.0200mol��L-1��31.0mL��10-3L��mL-1��CaC2O4�����ʵ���Ϊ0.0200mol��L-1��31.0��10-3L��5/2=0.00155mol
����CaO2����Ca2+�����ʵ���Ϊ��
n��Ca2+��=
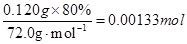
CaO�����ʵ���Ϊ0.00155mol-0.00133mol
m��CaO��=��0.00155mol-0.00133mol����56.0g��mol-1
=0.012g
m��H2O��=0.120g-0.120g��80.0��-0.012g=0.012g
CaO2��H2O�����ʵ���֮��Ϊ
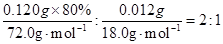
��
���֢������������ڴ�����ʽ��
CaO2��xH2O��CaC2O4��xH2O
��72+18x��g 1mol
0.120g 1.55��10-3mol
��0.120gȫ������CaO2��xH2O����������Ʒ�С�ͨ�����в���CaO����CaO2��xH2O��CaC2O4�����ϵʽҲ�Ǵ��ģ�CaC2O4�����ʵ�������Һ��ȫ��Ca2+�����ʵ�������������CaO2��������Ca2+�����ʵ�����
���֢�����������������Ʒ��CaO2������������80������Ϊ�ᾧˮ�����к�CaO2�������������������㣬����ʧ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
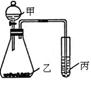
 ��1��ͼ�Т�Ϊ ����Ϊ ��
��1��ͼ�Т�Ϊ ����Ϊ �� X+H2 ��Y+NaOH��G+W+H2O
X+H2 ��Y+NaOH��G+W+H2O