��Ŀ����
��1����ͬѧ��ij��ʵ���д�Լ��Ҫ0.10mol/L������������Һ900mL������ʱ�漰�����²�����
������������ƿ����ʱ���ܽ���Һ����̶����⣩��ʹ��Һ��ֻ��
������ƽ�����������������ƹ���
�۴��ձ��е���Һ��ȴ���ز�����С�ĵ�ע��
�ܽ�����ƿ��ƿ���Ǻã��������µߵ���ҡ��
�ݽ����ƺõ���Һ�������ྻ���Լ�ƿ�У����ñ�ǩ����
����ʢ��NaOH�������У���������������ˮʹ������ȫ�ܽ�
�߸���
��������ˮϴ���ձ��ڱ�2-3�Σ���ÿ��ϴ�Ӻ����ҺҲ��ע������ƿ��
��������ƿ��ע������ˮ��ֱ������ƿ�е�Һ��ӽ�����ƿ�̶ȴ�1cm-2cm��
��Э����ͬѧ����ɲ����еĿ��ಿ�֣���������ѡ����ѡ����ȷ�IJ���˳����ȷ�IJ���˳����
A���ڢޢۢܢ٢ߢ���B���ڢޢۢ�٢�ߢܢ�
C���ڢۢ٢ܢޢߢ���D���ڢۢ٢ޢߢ��ܢ�
��2������ƿ�ϱ���
��3�����в�����ʹ������Һ���ʵ���Ũ��ƫ�͵���
A�����ݡ��Ӹǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶�
B������ʱ������ƿ�м�����ˮ�������̶��ߣ�����������
C��С�ձ���������û��ϴ��
D���۲�����ƿ�̶�ʱ����
E����������ƿ�У�������������ˮ
F����δ��������ʱ����������Һת��������ƿ����
G������ʱ�ѹ۲쵽NaOH��ˮ
��4������NaOH����ʱ��ע�������������⣺
����ΪNaOH���и�ʴ�ԣ����Գ���ʱ����ע��ѡ��
�ڳ�������Ѹ�٣�Ŀ����
��5�����ݵ���ȷ������
������������ƿ����ʱ���ܽ���Һ����̶����⣩��ʹ��Һ��ֻ��
������ƽ�����������������ƹ���
4.0
4.0
g�۴��ձ��е���Һ��ȴ���ز�����С�ĵ�ע��
1000mL����ƿ
1000mL����ƿ
���ܽ�����ƿ��ƿ���Ǻã��������µߵ���ҡ��
�ݽ����ƺõ���Һ�������ྻ���Լ�ƿ�У����ñ�ǩ����
����ʢ��NaOH�������У���������������ˮʹ������ȫ�ܽ�
�߸���
��ͷ�ι�
��ͷ�ι�
�μ�����ˮ����Һ�İ�Һ��������̶���������������ˮϴ���ձ��ڱ�2-3�Σ���ÿ��ϴ�Ӻ����ҺҲ��ע������ƿ��
��������ƿ��ע������ˮ��ֱ������ƿ�е�Һ��ӽ�����ƿ�̶ȴ�1cm-2cm��
��Э����ͬѧ����ɲ����еĿ��ಿ�֣���������ѡ����ѡ����ȷ�IJ���˳����ȷ�IJ���˳����
B
B
��A���ڢޢۢܢ٢ߢ���B���ڢޢۢ�٢�ߢܢ�
C���ڢۢ٢ܢޢߢ���D���ڢۢ٢ޢߢ��ܢ�
��2������ƿ�ϱ���
�٢ۢ�
�٢ۢ�
������ţ� ���¶� ��Ũ�� ���ݻ� ��ѹǿ �ݿ̶��� ��3�����в�����ʹ������Һ���ʵ���Ũ��ƫ�͵���
ABCDG
ABCDG
��A�����ݡ��Ӹǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶�
B������ʱ������ƿ�м�����ˮ�������̶��ߣ�����������
C��С�ձ���������û��ϴ��
D���۲�����ƿ�̶�ʱ����
E����������ƿ�У�������������ˮ
F����δ��������ʱ����������Һת��������ƿ����
G������ʱ�ѹ۲쵽NaOH��ˮ
��4������NaOH����ʱ��ע�������������⣺
����ΪNaOH���и�ʴ�ԣ����Գ���ʱ����ע��ѡ��
�ձ�
�ձ�
_ʢװNaOH���壻�ڳ�������Ѹ�٣�Ŀ����
��ֹ����ʱ�������NaOH��ˮ��������տ����еĶ�����̼������
��ֹ����ʱ�������NaOH��ˮ��������տ����еĶ�����̼������
����5�����ݵ���ȷ������
��ˮ����̶���1��2cmʱ�����ý�ͷ�ιܵμ�ˮ��Һ�棨��Һ����͵㣩��̶�������
��ˮ����̶���1��2cmʱ�����ý�ͷ�ιܵμ�ˮ��Һ�棨��Һ����͵㣩��̶�������
����������1������900mL��Һ����Ҫѡ��1000mL����ƿ�����Ƶ���ҺΪ1000mL 0.10mol/L������������Һ��
�ڸ���1000mL 0.10mol/L������������Һ���������Ƶ����ʵ���������������Ƶ�������
�۽���ȴ�������������Һת�Ƶ�1000mL����ƿ�У�
�������Ҫʹ�ý�ͷ�ιܽ��ж��ݣ�
��������һ�����ʵ���Ũ����Һ�IJ����������
��2����������ƿ�ϱ����ݻ����̶��ߺ��¶���ɣ�
��3������cB=
�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���nB����Һ�����V����ģ���nB������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����nB������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��
��4�������������ƾ��и�ʴ�ԡ��׳��⡢�����������̼��Ӧ������
��5����������һ�����ʵ���Ũ�ȵ���Һ����ȷ���ݷ������
�ڸ���1000mL 0.10mol/L������������Һ���������Ƶ����ʵ���������������Ƶ�������
�۽���ȴ�������������Һת�Ƶ�1000mL����ƿ�У�
�������Ҫʹ�ý�ͷ�ιܽ��ж��ݣ�
��������һ�����ʵ���Ũ����Һ�IJ����������
��2����������ƿ�ϱ����ݻ����̶��ߺ��¶���ɣ�
��3������cB=
| nB |
| V |
��4�������������ƾ��и�ʴ�ԡ��׳��⡢�����������̼��Ӧ������
��5����������һ�����ʵ���Ũ�ȵ���Һ����ȷ���ݷ������
����⣺��1��ʵ����û��900mL����ƿ��������Һʱ��Ҫѡ��1000mL����ƿ��
��1000mL 0.10mol/L������������Һ���������Ƶ����ʵ���Ϊ��0.10mol/L��1L=0.1mol����Ҫ�������Ƶ�����Ϊ��40g/mol��0.1mol=4.0g��
�ʴ�Ϊ��4.0��
�۴��ձ��е���Һ��ȴ���ز�����С�ĵ�ע��1000mL����ƿ�У�
�ʴ�Ϊ��1000mL����ƿ��
��������ƿ��ע������ˮ��ֱ������ƿ�е�Һ��ӽ�����ƿ�̶ȴ�1cm-2cm����Ȼ����ý�ͷ�ιܶ��ݣ�
�ʴ�Ϊ����ͷ�ιܣ�
����һ�����ʵ���Ũ�ȵ���Һ�IJ���Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���ȷ�IJ���˳���ǣ��ڢޢۢ�٢�ߢܢݣ�
��ѡB��
��2������ƿΪ��������������ƿ�ϱ����ݻ��Ϳ̶��ߣ��¶�Ӱ����Һ�������������Һ��Ҫ�����¼�С������ƿ�ϻ������¶ȣ�
��ѡ�٢ۢݣ�
��3��A�����ݡ��Ӹǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȣ��������Ƶ���Һ���ƫ����cB=
�ɵã����Ƶ���ҺŨ��ƫ�ͣ���A��ȷ��
B������ʱ������ƿ�м�����ˮ�������̶��ߣ��������������������Ƶ���Һ���ƫ����cB=
�ɵã���ҺŨ��ƫ�ͣ���B��ȷ��
C��С�ձ���������û��ϴ�ӣ��������Ƶ���Һ�����ʵ����ʵ�����С������cB=
�ɵã���Һ��Ũ��ƫ�ͣ���C��ȷ��
D���۲�����ƿ�̶�ʱ���ӣ����¼��������ˮ���ƫ����cB=
�ɵã���Һ��Ũ��ƫ�ͣ���D��ȷ��
E����������ƿ�У�������������ˮ�������ʵ����ʵ�������Һ�����û��Ӱ�죬��Ӱ�����ƽ������E����
F����δ��������ʱ����������Һת��������ƿ���ݣ��ȵ���Һ���ƫ����ȴ����Һ���ƫС������cB=
�ɵã���Һ��Ũ��ƫ�ߣ���F����
G������ʱ�ѹ۲쵽NaOH��ˮ�����������������Ƶ����ʵ���ƫС������cB=
�ɵã���Һ��Ũ��ƫ�ͣ���G��ȷ��
��ѡABCDG��
��4�����������ƾ��и�ʴ�ԣ�����ʱ��Ҫ�����ձ��У��ʴ�Ϊ���ձ���������������������
�����ڳ���ʱ�����NaOH��ˮ��������տ����еĶ�����̼�����ʣ����Գ�������Ѹ�٣�
�ʴ�Ϊ����ֹ����ʱ�������NaOH��ˮ��������տ����еĶ�����̼�����ʣ�
��5������һ�����ʵ���Ũ����Һʱ�����ݵ���ȷ����Ϊ����ˮ����̶���1��2cmʱ�����ý�ͷ�ιܵμ�ˮ��Һ�棨��Һ����͵㣩��̶������У�
�ʴ�Ϊ����ˮ����̶���1��2cmʱ�����ý�ͷ�ιܵμ�ˮ��Һ�棨��Һ����͵㣩��̶������У�
��1000mL 0.10mol/L������������Һ���������Ƶ����ʵ���Ϊ��0.10mol/L��1L=0.1mol����Ҫ�������Ƶ�����Ϊ��40g/mol��0.1mol=4.0g��
�ʴ�Ϊ��4.0��
�۴��ձ��е���Һ��ȴ���ز�����С�ĵ�ע��1000mL����ƿ�У�
�ʴ�Ϊ��1000mL����ƿ��
��������ƿ��ע������ˮ��ֱ������ƿ�е�Һ��ӽ�����ƿ�̶ȴ�1cm-2cm����Ȼ����ý�ͷ�ιܶ��ݣ�
�ʴ�Ϊ����ͷ�ιܣ�
����һ�����ʵ���Ũ�ȵ���Һ�IJ���Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���ȷ�IJ���˳���ǣ��ڢޢۢ�٢�ߢܢݣ�
��ѡB��
��2������ƿΪ��������������ƿ�ϱ����ݻ��Ϳ̶��ߣ��¶�Ӱ����Һ�������������Һ��Ҫ�����¼�С������ƿ�ϻ������¶ȣ�
��ѡ�٢ۢݣ�
��3��A�����ݡ��Ӹǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȣ��������Ƶ���Һ���ƫ����cB=
| nB |
| V |
B������ʱ������ƿ�м�����ˮ�������̶��ߣ��������������������Ƶ���Һ���ƫ����cB=
| nB |
| V |
C��С�ձ���������û��ϴ�ӣ��������Ƶ���Һ�����ʵ����ʵ�����С������cB=
| nB |
| V |
D���۲�����ƿ�̶�ʱ���ӣ����¼��������ˮ���ƫ����cB=
| nB |
| V |
E����������ƿ�У�������������ˮ�������ʵ����ʵ�������Һ�����û��Ӱ�죬��Ӱ�����ƽ������E����
F����δ��������ʱ����������Һת��������ƿ���ݣ��ȵ���Һ���ƫ����ȴ����Һ���ƫС������cB=
| nB |
| V |
G������ʱ�ѹ۲쵽NaOH��ˮ�����������������Ƶ����ʵ���ƫС������cB=
| nB |
| V |
��ѡABCDG��
��4�����������ƾ��и�ʴ�ԣ�����ʱ��Ҫ�����ձ��У��ʴ�Ϊ���ձ���������������������
�����ڳ���ʱ�����NaOH��ˮ��������տ����еĶ�����̼�����ʣ����Գ�������Ѹ�٣�
�ʴ�Ϊ����ֹ����ʱ�������NaOH��ˮ��������տ����еĶ�����̼�����ʣ�
��5������һ�����ʵ���Ũ����Һʱ�����ݵ���ȷ����Ϊ����ˮ����̶���1��2cmʱ�����ý�ͷ�ιܵμ�ˮ��Һ�棨��Һ����͵㣩��̶������У�
�ʴ�Ϊ����ˮ����̶���1��2cmʱ�����ý�ͷ�ιܵμ�ˮ��Һ�棨��Һ����͵㣩��̶������У�
���������⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ���������������Ŀ�Ѷ��еȣ����������ǿ�������߿���ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������������ѵ�����������������nB=
�ɵã�������ʱ�ؼ�Ҫ�����ƹ���������nB��V�����ı仯��
| nB |
| V |

��ϰ��ϵ�д�
�����Ŀ
ijʵ��С����������к͵ζ����ⶨʳ����������g/100mL��������������뱾ʵ�鲢�ش�������⣮���й�ʵ��ҩƷΪ������ʳ�ð״���Ʒ500mL��0.1000mol/LNaOH����Һ������ˮ��0.1%������Һ��0.1%��̪��Һ��0.1%ʯ����Һ����
��ʵ�鲽�裺
��1���õζ�����ȡ10mL���۰״���Ʒ������100mL����ƿ�У�������ˮ����г�ȥCO2��Ѹ����ȴ����ϡ�����̶��ߣ�ҡ�ȼ��ô���ʳ����Һ��
��2������ʽ�ζ���ȡ����ʳ����Һ20.00mL��______�У�
��3��ʢװ��NaOH��Һ�����ú�ȡ���ݣ���¼ΪNaOH����Һ����ij�������
��4���ζ�������¼NaOH���ն������ظ��ζ�2-3�Σ�
��ʵ���¼�����ݴ���
| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.98 | 15.20 | 15.12 | 15.95 |
| V��NaOH��/mL�����ģ� | 14.98 | 15.00 | 15.02 | 15.95 |
���������ۣ�
��1����ͬѧ�ڴ������ݹ����м���ã�V��NaOH����ƽ�����ģ�=1/4��14.98+15.00+15.02+15.95��mL=15.24mL��
�Է������ļ����Ƿ�����������������˵�����ɣ�______��
��2����ͬѧ��0.1000mol/LNaOH��Һ�ζ���һ���۰״���Ʒ��Һʱ���ζ�������ʹ��pH�ƽ���Һ��pH�仯�����¼���±���
| V��NaOH��/mL | 0.00 | 10.00 | 18.00 | 19.80 | 19.98 | 20.00 | 20.02 | 20.20 | 22.00 |
| ��ҺpH | 2.88 | 4.70 | 5.70 | 6.74 | 7.74 | 8.72 | 9.70 | 10.70 | 11.70 |
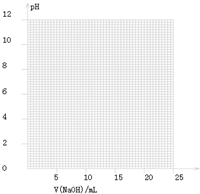
���ɱ���ͼ��֪������������Χ����0.1%���ڣ�pHͻ�䣨�ζ�ͻԾ����ΧΪ______�����Կ�ѡ��______��ָʾ����
��������ָʾ���ı�ɫ��Χ
| ָʾ�� | ��ɫ�ķ�Χ��pH�� |
| ���� | 3.1��4.4 |
| ʯ�� | 5.0��8.0 |
| ��̪ | 8.2��10.0 |