��Ŀ����
��2009?������ģ��ijʵ��С����������к͵ζ����ⶨʳ����������g/100mL��������������뱾ʵ�鲢�ش�������⣮���й�ʵ��ҩƷΪ������ʳ�ð״���Ʒ500mL��0.1000mol/LNaOH����Һ������ˮ��0.1%������Һ��0.1%��̪��Һ��0.1%ʯ����Һ����
��ʵ�鲽�裺
��1���õζ�����ȡ10mL���۰״���Ʒ������100mL����ƿ�У�������ˮ����г�ȥCO2��Ѹ����ȴ����ϡ�����̶��ߣ�ҡ�ȼ��ô���ʳ����Һ��
��2������ʽ�ζ���ȡ����ʳ����Һ20.00mL��
��3��ʢװ��NaOH��Һ�����ú�ȡ���ݣ���¼ΪNaOH����Һ����ij�������
��4���ζ�������¼NaOH���ն������ظ��ζ�2-3�Σ�
��ʵ���¼�����ݴ���
��c����Ʒ��/moL?L-1=
���������ۣ�
��1����ͬѧ�ڴ������ݹ����м���ã�V��NaOH����ƽ�����ģ�=1/4��14.98+15.00+15.02+15.95��mL=15.24mL��
�Է������ļ����Ƿ�����������������˵�����ɣ�
��2����ͬѧ��0.1000mol/LNaOH��Һ�ζ���һ���۰״���Ʒ��Һʱ���ζ�������ʹ��pH�ƽ���Һ��pH�仯�����¼���±���
��������pH-V��NaOH��ͼ��
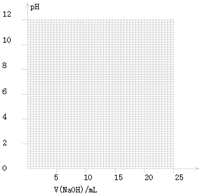
���ɱ���ͼ��֪������������Χ����0.1%���ڣ�pHͻ�䣨�ζ�ͻԾ����ΧΪ
��������ָʾ���ı�ɫ��Χ
��ʵ�鲽�裺
��1���õζ�����ȡ10mL���۰״���Ʒ������100mL����ƿ�У�������ˮ����г�ȥCO2��Ѹ����ȴ����ϡ�����̶��ߣ�ҡ�ȼ��ô���ʳ����Һ��
��2������ʽ�ζ���ȡ����ʳ����Һ20.00mL��
��ƿ
��ƿ
�У���3��ʢװ��NaOH��Һ�����ú�ȡ���ݣ���¼ΪNaOH����Һ����ij�������
��4���ζ�������¼NaOH���ն������ظ��ζ�2-3�Σ�
��ʵ���¼�����ݴ���
| �ζ����� ʵ������ |
1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.98 | 15.20 | 15.12 | 15.95 |
| V��NaOH��/mL�����ģ� | 14.98 | 15.00 | 15.02 | 15.95 |
0.75mol/L
0.75mol/L
����Ʒ������g/100mL=4��g/100ml
4��g/100ml
�����������ۣ�
��1����ͬѧ�ڴ������ݹ����м���ã�V��NaOH����ƽ�����ģ�=1/4��14.98+15.00+15.02+15.95��mL=15.24mL��
�Է������ļ����Ƿ�����������������˵�����ɣ�
����������Ϊ������������ǰ3�����ϴ����쳣ֵ��Ӧ��ȥ
����������Ϊ������������ǰ3�����ϴ����쳣ֵ��Ӧ��ȥ
����2����ͬѧ��0.1000mol/LNaOH��Һ�ζ���һ���۰״���Ʒ��Һʱ���ζ�������ʹ��pH�ƽ���Һ��pH�仯�����¼���±���
| V��NaOH��/mL | 0.00 | 10.00 | 18.00 | 19.80 | 19.98 | 20.00 | 20.02 | 20.20 | 22.00 |
| ��ҺpH | 2.88 | 4.70 | 5.70 | 6.74 | 7.74 | 8.72 | 9.70 | 10.70 | 11.70 |

���ɱ���ͼ��֪������������Χ����0.1%���ڣ�pHͻ�䣨�ζ�ͻԾ����ΧΪ
7.74��9.70
7.74��9.70
�����Կ�ѡ����̪
��̪
��ָʾ������������ָʾ���ı�ɫ��Χ
| ָʾ�� | ��ɫ�ķ�Χ��pH�� |
| ���� | 3.1��4.4 |
| ʯ�� | 5.0��8.0 |
| ��̪ | 8.2��10.0 |
�������ζ�������Һ�ڵζ����У�����Һʢ����ƿ�У�
����ͼ�����ݽ�ϴ�����������ư���1��1��Ӧ������Ʒ��ҺŨ�ȣ�����Ũ�ȼ�����Ʒ��������
������ͼ����������㷨�����仯���ߣ�
�ڸ��ݻ���ͼ������жϣ�pHͻ�䣨�ζ�ͻԾ��������PHͻ����ָʾ����ɫ��Χѡ����Ҫ��ָʾ����
����ͼ�����ݽ�ϴ�����������ư���1��1��Ӧ������Ʒ��ҺŨ�ȣ�����Ũ�ȼ�����Ʒ��������
������ͼ����������㷨�����仯���ߣ�
�ڸ��ݻ���ͼ������жϣ�pHͻ�䣨�ζ�ͻԾ��������PHͻ����ָʾ����ɫ��Χѡ����Ҫ��ָʾ����
����⣺������ʽ�ζ���ȡ����ʳ����Һ20.00mL����ƿ�У��ʴ�Ϊ����ƿ��
������ʵ�����ݽ�Ϸ�ӦCH3COOH+NaOH=CH3COONa+H2O��ʵ��1��2��3ƽ����������������Һ���=
=15.00ml��c����Ʒ��V����Ʒ��=c��NaOH��V��NaOH��
c����Ʒ����20.00ml=0.1000mol/L��15.00ml
c����Ʒ��=0.75mol/L��
��Ʒ������g/100mL=
��100ml=4.5g/100ml��
�ʴ�Ϊ��0.75mo/L��4.5g/100ml��
��1����ͬѧ�ڴ������ݹ����еļ��㲻��������Ϊ��4��ʵ���������̫��Ӧ��ȥ��
�ʴ�Ϊ������������Ϊ������������ǰ3�����ϴ����쳣ֵ��Ӧ��ȥ��
��2��������ͼ�����ݰ�����㷨����ͼ�� ���ʴ�Ϊ��
���ʴ�Ϊ��
������ͼ���ͼ�����ݷ�����֪PHͻ����7.74��9.70���������������ǡ�÷�Ӧ���ɵĴ�������Һ�ʼ��ԣ���̪PH��ɫ��ΧΪ8.2��10.0������ָʾ��ѡ���̪�Ϻõ��жϷ�Ӧ�յ㣻
�ʴ�Ϊ��7.74��9.70����̪��
������ʵ�����ݽ�Ϸ�ӦCH3COOH+NaOH=CH3COONa+H2O��ʵ��1��2��3ƽ����������������Һ���=
| 14.98+15+15.02 |
| 3 |
c����Ʒ����20.00ml=0.1000mol/L��15.00ml
c����Ʒ��=0.75mol/L��
��Ʒ������g/100mL=
| 0.75mol��60g/mol |
| 1000ml |
�ʴ�Ϊ��0.75mo/L��4.5g/100ml��
��1����ͬѧ�ڴ������ݹ����еļ��㲻��������Ϊ��4��ʵ���������̫��Ӧ��ȥ��
�ʴ�Ϊ������������Ϊ������������ǰ3�����ϴ����쳣ֵ��Ӧ��ȥ��
��2��������ͼ�����ݰ�����㷨����ͼ��
 ���ʴ�Ϊ��
���ʴ�Ϊ��
������ͼ���ͼ�����ݷ�����֪PHͻ����7.74��9.70���������������ǡ�÷�Ӧ���ɵĴ�������Һ�ʼ��ԣ���̪PH��ɫ��ΧΪ8.2��10.0������ָʾ��ѡ���̪�Ϻõ��жϷ�Ӧ�յ㣻
�ʴ�Ϊ��7.74��9.70����̪��
���������⿼��������к͵ζ�ʵ��IJ�������ͼ���Ӧ�ã���Ӧ�յ��ͼ����ƣ��յ��жϣ�ָʾ����ѡ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ��2009?������ģ����������������ǵڶ�������Ӷ�����أ���������ʹ������Ӷ�������ڴ��綯�������϶������ȴ�������Ӧ������ռ��������λ����ط�ӦʽΪ��Li1-xMnO4+LixC=LiMnO4+C�������й�˵������ȷ���ǣ�������
��2009?������ģ����������������ǵڶ�������Ӷ�����أ���������ʹ������Ӷ�������ڴ��綯�������϶������ȴ�������Ӧ������ռ��������λ����ط�ӦʽΪ��Li1-xMnO4+LixC=LiMnO4+C�������й�˵������ȷ���ǣ�������