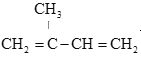Ő‚ńŅńŕ»›
°ĺŐ‚ńŅ°Ņ Į”Õ «Ļ§“ĶĶń—™“ļ£¨Õ®ĻżňŁŅ…“‘Ķ√ĶĹļ‹∂ŗ÷ō“™ĶńĽĮĻ§≤ķ∆∑°£

“—÷™£ļ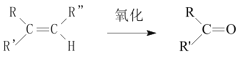 +
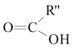
+
£®1£©BļÕAő™Õ¨ŌĶőÔ£¨BĶńĹŠĻĻľÚ Ĺő™____£¨∆šļ¨”–ĶńĻŔń‹ÕŇ√Ż≥∆ő™____°£
£®2£©∑ī”¶ĘŔĶńĽĮ—ß∑Ĺ≥Ő Ĺő™___£¨∆š∑ī”¶ņŗ–Õő™____°£
£®3£©–ī≥Ųľž—ťC3H5Cl÷–ļ¨”–ĶńCl‘≠◊”Ķń∑Ĺ∑®____°£
£®4£©CĶńĹŠĻĻľÚ Ĺő™___°£
£®5£©∑ī”¶Ę‹ĶńĽĮ—ß∑Ĺ≥Ő Ĺő™_____°£
£®6£©…Ťľ∆“ĽŐű”…““Ō©ő™‘≠ŃŌ÷∆ĪłDĶńļŌ≥…¬∑ŌŖ£®∆šňŻőřĽķ‘≠ŃŌ»ő—°£©°£___
ļŌ≥…¬∑ŌŖŃų≥ŐÕľ ĺņż»ÁŌ¬£ļCH3CH2OH![]() CH2=CH2
CH2=CH2![]() BrH2C-CH2Br°£
BrH2C-CH2Br°£
°ĺīūįł°ŅCH2=CHCH3 ŐľŐľň꾣 CH2=CH2+H2O![]() CH3CH2OH ľ”≥…∑ī”¶ »°—ý£¨Ō»ľ”»ŽNaOH»‹“ļľ”»»£¨ņš»īļůľ”»ŽŌ°ŌűňŠňŠĽĮ£¨‘Ŕľ”»ŽAgNO3»‹“ļ£¨”–į◊…ę≥ŃĶŪ…ķ≥…‘Úļ¨”–Cl‘≠◊”£¨∑Ů‘Ú≤Ľļ¨ HCOO-COOH CH2=CHCH2Cl+NaOH
CH3CH2OH ľ”≥…∑ī”¶ »°—ý£¨Ō»ľ”»ŽNaOH»‹“ļľ”»»£¨ņš»īļůľ”»ŽŌ°ŌűňŠňŠĽĮ£¨‘Ŕľ”»ŽAgNO3»‹“ļ£¨”–į◊…ę≥ŃĶŪ…ķ≥…‘Úļ¨”–Cl‘≠◊”£¨∑Ů‘Ú≤Ľļ¨ HCOO-COOH CH2=CHCH2Cl+NaOH![]() CH2=CHCH2OH+NaCl CH2=CH2
CH2=CHCH2OH+NaCl CH2=CH2![]() CH3CH2OH
CH3CH2OH
CH2=CH2![]() BrCH2-CH2Br
BrCH2-CH2Br![]() HOCH2-CH2OH
HOCH2-CH2OH![]()
![]()
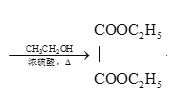
°ĺĹ‚őŲ°Ņ
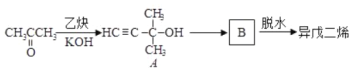
”…Ńų≥ŐÕľŅ…÷™Aő™““Ō©£¨”Žňģľ”≥…Ķ√““īľ£¨”ŽC∑ī”¶…ķ≥…D£¨Cő™ HCOO-COOH£Ľ
Bő™CH2=CHCH3£¨”Ž¬»∆Ý∑Ę…ķ»°īķ∑ī”¶Ķ√CH2=CHCH2Cl£¨‘Ŕ∑Ę…ķCH2=CHCH2Cl+NaOH![]() CH2=CHCH2OH+NaCl£¨Ķ√CH2=CHCH2OH£¨łýĺ›–ŇŌĘ—űĽĮ…ķ≥…C°£
CH2=CHCH2OH+NaCl£¨Ķ√CH2=CHCH2OH£¨łýĺ›–ŇŌĘ—űĽĮ…ķ≥…C°£
£®1£©Aő™““Ō©£¨BļÕAő™Õ¨ŌĶőÔ£¨”–3łŲŐľ£¨ňý“‘BĶńĹŠĻĻľÚ Ĺő™CH2=CHCH3£¨∆šļ¨”–ĶńĻŔń‹ÕŇ√Ż≥∆ő™ŐľŐľň꾣°£
£®2£©CH2=CH2ļÕH2O∑Ę…ķľ”≥…∑ī”¶£¨∑ī”¶ĘŔĶńĽĮ—ß∑Ĺ≥Ő Ĺő™ CH2=CH2+H2O![]() CH3CH2OH£¨∆š∑ī”¶ņŗ–Õő™ľ”≥…∑ī”¶°£
CH3CH2OH£¨∆š∑ī”¶ņŗ–Õő™ľ”≥…∑ī”¶°£
£®3£©C3H5Cl «Ļ≤ľŘĽĮļŌőÔ£¨‘ŕňģ÷–ń—ĶÁņŽ£¨“™Ō»ňģĹ‚≤ķ…ķ¬»ņŽ◊”£¨ľž—ťC3H5Cl÷–ļ¨”–ĶńCl‘≠◊”Ķń∑Ĺ∑®£ļ»°—ý£¨Ō»ľ”»ŽNaOH»‹“ļľ”»»£¨ņš»īļůľ”»ŽŌ°ŌűňŠňŠĽĮ£¨‘Ŕľ”»ŽAgNO3»‹“ļ£¨”–į◊…ę≥ŃĶŪ…ķ≥…‘Úļ¨”–Cl‘≠◊”£¨∑Ů‘Ú≤Ľļ¨°£
£®4£©Aő™““Ō©£¨”Žňģľ”≥…Ķ√““īľ£¨”ŽC∑ī”¶…ķ≥…D£¨Cő™ HCOO-COOH°£
£®5£©¬ĪīķŐĢ‘ŕľÓ–‘ŐűľĢŌ¬∑Ę…ķňģĹ‚∑ī”¶£¨Ę‹ĶńĽĮ—ß∑Ĺ≥Ő Ĺő™CH2=CHCH2Cl+NaOH![]() CH2=CHCH2OH+NaCl °£
CH2=CHCH2OH+NaCl °£
£®6£©““Ō©”ŽšŚľ”≥…£¨Ķ√BrCH2-CH2Br£¨‘ŕľÓ–‘ŐűľĢŌ¬ňģĹ‚≥…HOCH2-CH2OH£¨‘Ŕ—űĽĮĶ√““∂ĢňŠ£¨◊Óļůĺ≠ű•ĽĮ∑ī”¶Ķ√D£¨”…““Ō©ő™‘≠ŃŌ÷∆ĪłDĶńļŌ≥…¬∑ŌŖ
CH2=CH2![]() CH3CH2OH
CH3CH2OH
CH2=CH2![]() BrCH2-CH2Br
BrCH2-CH2Br![]() HOCH2-CH2OH
HOCH2-CH2OH![]()
![]()
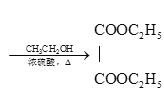 °£
°£

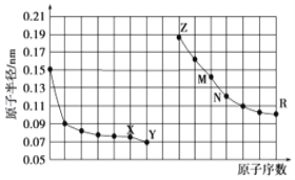
°ĺŐ‚ńŅ°ŅŌ¬ĪŪ «‘™ňō÷‹∆ŕĪŪĶń“Ľ≤Ņ∑÷£¨”√ĽĮ—ß”√”ÔĽōīūŌ¬Ń–ő Ő‚£ļ
ĘŮA | ĘÚA | ĘůA | ĘŰA | ĘűA | ĘŲA | ĘųA | |
∂Ģ | ĘŔ | Ęŕ | ĘŘ | ||||
»ż | Ę‹ | Ę› | Ęř | ĘŖ | Ęŗ |
£®1£©ĘŘĶń‘™ňō∑ŻļŇő™___£¨‘™ňōĘ‹”ŽĘŖ–ő≥…ĽĮļŌőÔĶńĶÁ◊” Ĺő™___°£
£®2£©Ī»ĹŌ‘™ňōĘŔļÕĘřĶń‘≠◊”įŽĺ∂īů–°£ļĘŔ___£®ŐÓ°į>°ĪĽÚ°į<°Ī£©Ęř
£®3£©‘™ňōĘřĶńņŽ◊”ĹŠĻĻ ĺ“‚Õľő™___°£
£®4£©‘™ňōĘŔļÕĘŗ–ő≥…ĶńĽĮļŌőÔ÷–ļ¨”–ĶńĽĮ—ßľŁő™___°£
£®5£©‘™ňōĘŕ°ĘĘ›Ķń◊ÓłŖľŘ—űĽĮőÔ∂‘”¶ĶńňģĽĮőÔ÷ģľš∑ī”¶ĶńņŽ◊”∑Ĺ≥Ő Ĺő™___°£
£®6£©Ļ§“Ķ…Ō“ĪŃ∂‘™ňōĘřĶńĶ•÷ ĶńĽĮ—ß∑Ĺ≥Ő Ĺő™___°£
°ĺŐ‚ńŅ°Ņ°į¬Őňģ«ŗ…ĹĺÕ «Ĺū…Ĺ“Ý…Ĺ°Ī£¨“Úīň—–ĺŅNOx°ĘSO2Ķ»īů∆ÝőŘ»ĺőÔĶńÕ◊…∆ī¶ņŪĺŖ”–÷ō“™“‚“Ś°£
(1)SO2ĶńŇŇ∑Ň÷ų“™ņī◊‘”ŕ√ļĶń»ľ…’£¨Ļ§“Ķ…Ō≥£”√įĪňģőŁ ’∑®ī¶ņŪő≤∆Ý÷–ĶńSO2°£
“—÷™őŁ ’Ļż≥Ő÷–ŌŗĻō∑ī”¶Ķń»»ĽĮ—ß∑Ĺ≥Ő Ĺ»ÁŌ¬£ļ
ĘŔSO2(g)+NH3°§H2O(aq)=NH4HSO3(aq) ¶§H1=a kJ/mol£Ľ
ĘŕNH3°§H2O(aq)+ NH4HSO3(aq)=(NH4)2SO3(aq)+H2O(l) ¶§H 2=b kJ/mol£Ľ
ĘŘ2(NH4)2SO3(aq)+O2(g)=2(NH4)2SO4(aq) ¶§H 3=c kJ/mol°£
‘Ú∑ī”¶2SO2(g)+4NH3°§H2O(aq)+O2(g)=2(NH4)2SO4(aq)+2H2O(l)Ķń¶§H =______kJ/mol°£
(2)»ľ√ļ∑ĘĶÁ≥ß≥£ņŻ”√∑ī”¶2CaCO3(s)+2SO2(g)+O2(g)![]() 2CaSO4(s)+2CO2(g) ¶§H =-681.8 kJ/mol∂‘√ļĹÝ––Õ—ŃÚī¶ņŪņīľű…ŔSO2ĶńŇŇ∑Ň°£∂‘”ŕł√∑ī”¶£¨‘ŕő¬∂»ő™T K Ī£¨ĹŤ÷ķīęł–∆ų≤‚Ķ√∑ī”¶‘ŕ≤ĽÕ¨ ĪľšĶ„…ŌłųőÔ÷ ĶńŇ®∂»»ÁŌ¬£ļ
2CaSO4(s)+2CO2(g) ¶§H =-681.8 kJ/mol∂‘√ļĹÝ––Õ—ŃÚī¶ņŪņīľű…ŔSO2ĶńŇŇ∑Ň°£∂‘”ŕł√∑ī”¶£¨‘ŕő¬∂»ő™T K Ī£¨ĹŤ÷ķīęł–∆ų≤‚Ķ√∑ī”¶‘ŕ≤ĽÕ¨ ĪľšĶ„…ŌłųőÔ÷ ĶńŇ®∂»»ÁŌ¬£ļ
Īľš/min Ň®∂»/mol/L | 0 | 10 | 20 | 30 | 40 | 50 |
O2 | 1.00 | 0.79 | 0.60 | 0.60 | 0.64 | 0.64 |
CO2 | 0 | 0.42 | 0.80 | 0.80 | 0.88 | 0.88 |
ĘŔ0~10 minńŕ£¨∆Ĺĺý∑ī”¶ňŔ¬ v(SO2)=___________mol/(L°§min)°£
Ęŕ30 minļů£¨÷ĽłńĪšń≥“ĽŐűľĢ£¨∑ī”¶÷ō–¬īÔĶĹ∆Ĺļ‚°£łýĺ›…ŌĪŪ÷–Ķń żĺ›Ň–∂Ō£¨łńĪšĶńŐűľĢŅ…ń‹ «___________£®ŐÓ◊÷ńł£©°£
A£ģľ”»Ž“Ľ∂®ŃŅĶń∑Ř◊īŐľňŠł∆ B£ģÕ®»Ž“Ľ∂®ŃŅĶńO2
C£ģ ĶĪňű–°»›∆ųĶńŐŚĽż D£ģľ”»ŽļŌ ĶńīŖĽĮľŃ
(3)NOxĶńŇŇ∑Ň÷ų“™ņī◊‘”ŕ∆Ż≥Ķő≤∆Ý£¨Ņ…≤…”√NSR(NOxīĘīśĽĻ‘≠)ĹÝ––ī¶ņŪ£¨NOxĶńīĘīśļÕĽĻ‘≠‘ŕ≤ĽÕ¨ Ī∂őĹĽŐśĹÝ––£¨»ÁÕľaňý ĺ°£

ĘŔÕ®ĻżBaOļÕBa(NO3)2ĶńŌŗĽ•◊™ĽĮ ĶŌ÷NOxĶńīĘīśļÕĽĻ‘≠°£īĘīśNOxĶńőÔ÷ «_________°£
ĘŕNOxīĘīś◊™ĽĮő™Ba(NO3)2Ļż≥Ő÷–£¨≤őľ”∑ī”¶ĶńNOļÕO2ĶńőÔ÷ ĶńŃŅ÷ģĪ»ő™_________°£
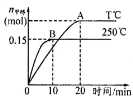
(4)”–»ňņŻ”√∑ī”¶C(s)+2NO(g)![]() N2(g)+CO2(g) ¶§H=34.0 kJ/mol£¨”√ĽÓ–‘ŐŅ∂‘NOĹÝ––őŁłĹ°£‘ŕ√‹Ī’»›∆ų÷–ľ”»Ž◊„ŃŅĶńCļÕ“Ľ∂®ŃŅĶńNO∆ÝŐŚ£¨Ī£≥÷ļ„—Ļ‘ŕ≤ĽÕ¨ő¬∂»Ō¬∑Ę…ķł√∑ī”¶£¨≤Ę∑÷Īū‘ŕt√Ž Ī≤‚Ķ√NOĶń◊™ĽĮ¬ £¨»ÁÕľňý ĺ£ļ
N2(g)+CO2(g) ¶§H=34.0 kJ/mol£¨”√ĽÓ–‘ŐŅ∂‘NOĹÝ––őŁłĹ°£‘ŕ√‹Ī’»›∆ų÷–ľ”»Ž◊„ŃŅĶńCļÕ“Ľ∂®ŃŅĶńNO∆ÝŐŚ£¨Ī£≥÷ļ„—Ļ‘ŕ≤ĽÕ¨ő¬∂»Ō¬∑Ę…ķł√∑ī”¶£¨≤Ę∑÷Īū‘ŕt√Ž Ī≤‚Ķ√NOĶń◊™ĽĮ¬ £¨»ÁÕľňý ĺ£ļ

ĘŔ”…ÕľŅ…÷™£¨1050 K«į∑ī”¶÷–NOĶń◊™ĽĮ¬ ňśő¬∂»…żłŖ∂Ý‘Ųīů£¨∆š‘≠“Úő™ _______________£Ľ‘ŕ1100 K Ī£¨CO2ĶńŐŚĽż∑÷ żő™___________°£
Ęŕ”√ń≥őÔ÷ Ķń∆Ĺļ‚∑÷—ĻīķŐś∆šőÔ÷ ĶńŃŅŇ®∂»“≤Ņ…“‘ĪŪ ĺĽĮ—ß∆Ĺļ‚≥£ ż£®ľ«◊ųKp£©£¨‘ŕ1050K1.1°Ń106 Pa Ī£¨ł√∑ī”¶ĶńĽĮ—ß∆Ĺļ‚≥£ żKp=______________________°£
[“—÷™£ļ∆ÝŐŚ∑÷—Ļ(P∑÷)=∆ÝŐŚ◊‹—Ļ(P)°ŃŐŚĽż∑÷ ż]