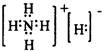��Ŀ����
ij������ˮ��Һ�����ܺ������������е������֣�K+��Al3+��Fe3+��Mg2+��Ba2+��N
��Cl-��C
��S
���ֱַ�ȡ100mL�����ȷ���Һ��������ʵ�飺
�ٵ�һ�ݼӹ���NaOH��Һ����ȣ�ֻ�ռ�������0.02mol���������ɣ�ͬʱ�õ���Һ�ף�
�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02g���壮
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65g���壮
�ش�
��1��һ�������ڵ�������
��2���ɢٿ�֪��������Ϊ
�ɢڿ�֪��������Ϊ
�ɢۿ�֪��������Ϊ
��3��K+�Ƿ���ڣ�
| H | - 4 |
| O | 2- 3 |
| O | 2- 4 |
�ٵ�һ�ݼӹ���NaOH��Һ����ȣ�ֻ�ռ�������0.02mol���������ɣ�ͬʱ�õ���Һ�ף�
�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02g���壮
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65g���壮
�ش�
��1��һ�������ڵ�������
Fe3+��Mg2+��Ba2+��CO32-
Fe3+��Mg2+��Ba2+��CO32-
�������ӷ��ţ���ͬ������2���ɢٿ�֪��������Ϊ
NH4+
NH4+
��Ũ��0.2 mol/L
0.2 mol/L
���ɢڿ�֪��������Ϊ
Al3+
Al3+
��Ũ��0.2 mol/L
0.2 mol/L
���ɢۿ�֪��������Ϊ
SO42-
SO42-
��Ũ��0.5 mol/L
0.5 mol/L
����3��K+�Ƿ���ڣ�
��
��
����ǡ��������������ݵ���غ㣬���������������С�������Ӹ��������������һ����K+����
���ݵ���غ㣬���������������С�������Ӹ��������������һ����K+����
����������һ�ݼӹ���NaOH��Һ����ȣ�ֻ�ռ�������0.02mol���������ɣ�ͬʱ�õ���Һ�ף������������ʷ����ƶϣ��������������������һ����NH4+���ӣ���������NH3���ʵ���Ϊ0.02mol����������֤����Һ��һ������Fe3+��Mg2+�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02g���壮���������̼��Ӧ�õ�������������Ba2+�����������ڹ��������������ɵ�ƫ���������ڷ�Ӧ�������������������ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65g���壮˵�������������ᣬ֤�������ᱵ������ԭ��Һ�к���SO42-���ж���Һ��һ��������Ba2+�������Һ��ͨ�����CO2�����ɰ�ɫ����ֻ���������������������������ˡ�ϴ�ӡ����պõ�1.02g����Ϊ��������ԭ��Һ�� ����Al3+��������ӹ����ж���Һ��һ��������CO32-�������Һ����غ��ж���Һ�еļ����Ӵ��ڣ�
����⣺��һ�ݼӹ���NaOH��Һ����ȣ�ֻ�ռ�������0.02mol���������ɣ�ͬʱ�õ���Һ�ף������������ʷ����ƶϣ��������������������һ����NH4+���ӣ���������NH3���ʵ���Ϊ0.02mol����������֤����Һ��һ������Fe3+��Mg2+�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02g���壮���������̼��Ӧ�õ�������������Ba2+�����������ڹ��������������ɵ�ƫ���������ڷ�Ӧ�������������������ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65g���壮˵�������������ᣬ֤�������ᱵ������ԭ��Һ�к���SO42-���ж���Һ��һ��������Ba2+�������Һ��ͨ�����CO2�����ɰ�ɫ����ֻ���������������������������ˡ�ϴ�ӡ����պõ�1.02g����Ϊ��������ԭ��Һ�� ����Al3+��������ӹ����ж���Һ��һ��������CO32-��һ�����е�����ΪNH4+ Al3+SO42-��
��1���������������жϣ�һ�������ڵ�����Ϊ��Fe3+��Mg2+��Ba2+��CO32-���ʴ�Ϊ��Fe3+��Mg2+��Ba2+��CO32-��
��2���ɢٿ�֪��������NH4+ ���ʵ���Ũ��=
=0.2 mol/L��
�ɢڿ�֪��������ΪAl3+�����������ˡ�ϴ�ӡ����պõ�1.02g����Ϊ���������ʵ���=
=0.01mol����Һ��Al3+�����ʵ���Ũ��=
=0.2 mol/L��
�ɢۿ�֪��������ΪSO42-���ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65g����Ϊ���ᱵ�����ʵ���=
=0.05mol��SO42- ���ʵ���Ũ��=
=0.5 mol/L���ʴ�Ϊ��NH4+��0.2 mol/L��Al3+��0.2 mol/L��SO42-��0.5 mol/L��
��3��������Һ�е���غ㣬���������������С�������Ӹ���������������ӵ����Ϊ0.8mol����������ֻ�����������ʱ�����Ϊ1mol������һ����K+���ڣ�
�ʴ�Ϊ���ǣ����ݵ���غ㣬���������������С�������Ӹ��������������һ����K+���ڣ�
��1���������������жϣ�һ�������ڵ�����Ϊ��Fe3+��Mg2+��Ba2+��CO32-���ʴ�Ϊ��Fe3+��Mg2+��Ba2+��CO32-��
��2���ɢٿ�֪��������NH4+ ���ʵ���Ũ��=
| 0.02mol |
| 0.1L |
�ɢڿ�֪��������ΪAl3+�����������ˡ�ϴ�ӡ����պõ�1.02g����Ϊ���������ʵ���=
| 1.02g |
| 102g/mol |
| 0.02mol |
| 0.1L |
�ɢۿ�֪��������ΪSO42-���ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65g����Ϊ���ᱵ�����ʵ���=
| 11.65 |
| 233g/mol |
| 0.05mol |
| 0.1L |
��3��������Һ�е���غ㣬���������������С�������Ӹ���������������ӵ����Ϊ0.8mol����������ֻ�����������ʱ�����Ϊ1mol������һ����K+���ڣ�
�ʴ�Ϊ���ǣ����ݵ���غ㣬���������������С�������Ӹ��������������һ����K+���ڣ�
���������⿼���˳������ӵļ��鷽�����������ʵ�Ӧ�ã���Ӧ������жϣ����ӹ���ķ���Ӧ�ã���ȷ�����ӡ���������ӡ�笠����ӵķ����жϺͼ����ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ