��Ŀ����
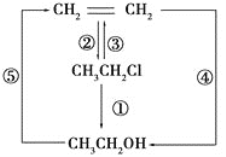
����Ŀ��������Ҵ���Ӧ��ȡ����������װ����ͼ��ʾ�����Թ������3mL�Ҵ���Ȼ��һ��ҡ����һ�������ؼ���2mLŨ�����2mL�����ᣬ�þƾ���С�ľ��ȵؼ���10min����������������������ͨ�뵽С�Թ�����Һ��Һ���ϡ��ش��������⣺
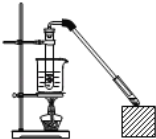
��1������Ĺ�������____��д���ƣ�����ȡ���������Ļ�ѧ����ʽΪ��___��
��2����ŨH2SO4��������____��
��С�Թ��е���Һ��____����������____��д��һ�ּ��ɣ���
�۳����ܲ�����Һ�����µ�Ŀ����____��
��4��ʵ���в�ȡ�ļ��ȷ�ʽ�ƣ����ּ��ȷ�ʽ�ĺô���Ҫ��____��___��
��5����Ӧ���ɵ��������������ܶȱ�ˮ___��������������С��������___��ζ��Ӧ�Ӹ÷�Һ©����___�����ţ������������÷֣���
a���²����� b���Ͽڵ��� c��������
��6����ʵ�������¶ȹ��ߣ��¶ȴ�170�棬����������Ҫ�л�������___�������ƣ���
���𰸡��Ȼ� CH3COOH +C2H5OH![]() CH3COOC2H5+H2O ��������ˮ�� ���ջӷ����Ҵ��������ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�� ��ֹ���� ˮԡ���� ���ڿ����¶� ʹ���Ⱦ��� С �� b ��ϩ
CH3COOC2H5+H2O ��������ˮ�� ���ջӷ����Ҵ��������ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�� ��ֹ���� ˮԡ���� ���ڿ����¶� ʹ���Ⱦ��� С �� b ��ϩ
��������
��1����������Ĺ�����Ϊ�Ȼ����ܺʹ�����������Ӧ������
��2��������Ӧ��Ũ���������Ϊ��������ˮ��������ñ���̼������Һ�������������������������Ҵ�����Ӧ���ᣬ���������ܽ�����á�
��3��ˮԡ���ȿ���ͨ������ˮԡ���¶Ƚ������Ʒ�Ӧ���¶ȣ������ƣ����Ƿ�Ӧ��ϵ�������������ȡ�
��4�����������ܶȲ�ˮС�����ϲ㣬��Һʱ���Ͽڵ�����
��5���Ҵ���Ũ��������¼��ȵ�170����Է�����ȥ��Ӧ������ϩ��
(1).����Ĺ�����Ϊ���Ȼ���������Ҵ���Ӧ��������������ˮ������ʽΪ��CH3COOH +C2H5OH![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
(2) �ٷ�Ӧ��Ũ���������Ϊ��������ˮ����
��С�Թ��е�Һ��ӦΪ����̼������Һ��������Ϊ���ջӷ����Ҵ��������ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ�
����Ϊ������Ҵ�����������ˮ�����Ե��ܲ�������Һ�����£�Ϊ�˷�ֹ���� ��
(3)ʵ���еļ��ȷ�ʽΪˮԡ���ȣ��ô��б��ڿ����¶ȡ�ʹ���Ⱦ��ȣ�
(4)�����������ܶȱ�ˮС������ζ����ҺʱӦ���²�Һ���������Ͽڵ�����
(5)�Ҵ���170��ʱ��Ũ����������·�����ȥ��Ӧ������ϩ��

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�����Ŀ������������Դ��Ϊ�����о��ȵ㡣CH3NH2��PbI2��HI�������ϳ�̫���ܵ�ص��������װ�Ǧ��(CH3NH3PbI3)����Ҫԭ�ϡ�
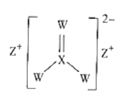
��1����ȡ�װ��ķ�ӦΪCH3OH(g)��NH3(g)![]() CH3NH2(g)��H2O(g) ��H����֪�÷�Ӧ����ػ�ѧ���ļ����������£�
CH3NH2(g)��H2O(g) ��H����֪�÷�Ӧ����ػ�ѧ���ļ����������£�
���ۼ� | C-O | H-O | N-H | C-N |
����/kJ��mol-1 | E1 | E2 | E3 | E4 |
�������Ȼ�ѧ����ʽ����H=___kJ��mol-1��
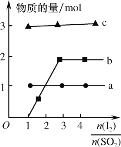
��2��������ˮú���ϳ�������Ӧ����״�����ӦΪCO(g)��2H2(g)![]() CH3OH(g)��H��0����һ�������£���1molCO��2molH2ͨ���ܱ������н��з�Ӧ�����ı�ijһ�������(�¶Ȼ�ѹǿ)ʱ��CH3OH�����������(CH3OH)�仯������ͼ��ʾ��
CH3OH(g)��H��0����һ�������£���1molCO��2molH2ͨ���ܱ������н��з�Ӧ�����ı�ijһ�������(�¶Ȼ�ѹǿ)ʱ��CH3OH�����������(CH3OH)�仯������ͼ��ʾ��
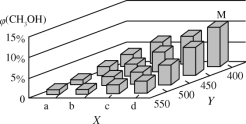
��ƽ��ʱ��M��CH3OH���������Ϊ10%����CO��ת����Ϊ___��
��ͼ��Y���ʾ�¶ȣ����жϵ�������__��
��3����������������Ǧ������ᷴӦ�Ʊ�����PbI2������Ӧ������amolPbI2����ת�Ƶ��ӵ����ʵ���Ϊ___��
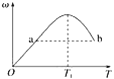
��4������������ͨ���ˮ�Ʊ�HI�ķ�Ӧ������ͼ��ʾ���䷴Ӧԭ��Ϊ��SO2��I2��2H2O=3H����HSO4-��2I-��I2��I-![]() I3-��ͼ������a��b�ֱ����������__��__���������ţ�����ͼ֪Ҫ��ߵ�Ļ�ԭ�ʣ��������¶��⣬�����Բ�ȡ�Ĵ�ʩ��___��
I3-��ͼ������a��b�ֱ����������__��__���������ţ�����ͼ֪Ҫ��ߵ�Ļ�ԭ�ʣ��������¶��⣬�����Բ�ȡ�Ĵ�ʩ��___��